The C-terminal regulatory domain of IPMS enzyme maintains leucine homeostasis by bypassing a hidden negative feedback loop in plants
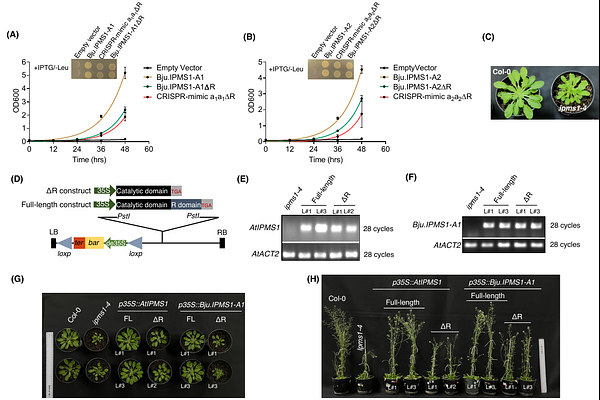
The C-terminal regulatory domain of IPMS enzyme maintains leucine homeostasis by bypassing a hidden negative feedback loop in plants
Varghese, M.; Kumar, R.; Sharma, A.; Lone, A.; Gershenzon, J.; Bisht, N. C.
AbstractIn the leucine biosynthesis pathway, homeostasis is achieved through a feedback regulatory mechanism facilitated by binding of the end-product Leu at the C-terminal regulatory domain of the first committed enzyme, isopropylmalate synthase (IPMS). In-vitro studies showed that removal of the regulatory domain abolishes the feedback regulation on plant IPMS while retaining its catalytic activity. However, the physiological consequences and underlying molecular regulation upon removal of the IPMS C-terminal domain on the Leu flux have not been previously explored in plants. Here, we show that in the absence of its regulatory domain, an unexpected alternative regulatory loop acts to control plant IPMS catalysis. Removal of IPMS regulatory domain using CRISPR/Cas9 significantly reduced the formation of end-product Leu in-planta, but increased the levels of Leu pathway intermediates. Additionally, delayed growth was observed when IPMS devoid of regulatory domain was introduced into IPMS-null mutants of E. coli and Arabidopsis. Combining the metabolomic and biochemical analysis, we found that the Leu pathway intermediate, -ketoisocaproate, was a competitive inhibitor of IPMS with a truncated regulatory domain. Thus, we demonstrate that the C-terminal regulatory domain of IPMS is biologically favored since it maintains Leu homeostasis while bypassing the possibility of competitive inhibition by a pathway intermediate.