Younger is Better But Only for Males: Social Behavioral Development Following Juvenile Traumatic Brain Injury to the Prefrontal Cortex
Younger is Better But Only for Males: Social Behavioral Development Following Juvenile Traumatic Brain Injury to the Prefrontal Cortex
Shonka, S.; Hylin, M. J.
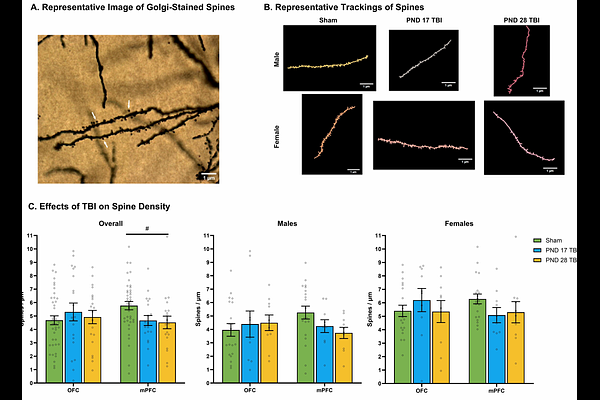
AbstractJuvenile traumatic brain injury (jTBI) is associated with persistent social impairments, particularly when injury occurs early in development. The prefrontal cortex (PFC) is especially vulnerable to injury due to its late maturation and location, and developmental disruptions during key phases such as synaptic pruning or myelination may result in long-term behavioral deficits. This study investigated how the age at injury and biological sex influenced the development of social behavior and frontal cortical plasticity. Using a controlled cortical impact model of a bilateral medial PFC (mPFC) injury, we compared injuries sustained on postnatal day (PND) 17 or 28--approximating toddlerhood and middle childhood, respectively. Social behaviors were assessed longitudinally during pre-puberty, puberty, and young adulthood. Play behavior, sociability, social memory, social dominance, and aggression were evaluated, and morphological analyses examined dendritic complexity in the orbitofrontal cortex (OFC) and mPFC using Golgi-Cox staining, and myelin integrity across the mPFC-OFC-amygdala circuit using Luxol-fast blue staining. We hypothesized that PND 17 injuries would result in greater social deficits and increased aggression compared to PND 28 injuries and shams, with males showing more severe impairments than females. Results partially supported these predictions. While jTBI had no effect on general play engagement, it did alter play initiation: PND 28 injury increased play initiation in both sexes, while PND 17 TBI injury delayed normal play development in females. Injuries had no significant impact on sociability or social memory. However, PND 28 injury increased social dominance and aggression in adulthood, with sex moderating these effects. Specifically, PND 17 injury decreased aggression in males and increased it in females. Childhood play behaviors predicted adult aggression, particularly in PND 28-injured animals, and these relationships were moderated by injury and sex. Despite the behavioral findings, histological analyses revealed no significant group differences in dendritic complexity or myelination, though effect sizes suggested decreased dendritic arborization and increased myelin in PND 28-injured animals. These findings highlight age- and sex-dependent vulnerability in the development of social behavior following jTBI. Contrary to expectations, early injury had more pronounced effects in females, while later injury was more detrimental for males. The divergence in behavioral outcomes despite limited histological differences suggests complex, possibly circuit-specific, mechanisms underlying these effects. This study underscores the importance of considering both sex and developmental timing in jTBI research and supports the need for longitudinal models to capture evolving behavioral trajectories.