Provision of Preferred Nutrients to Macrophages Enables Salmonella to Replicate Intracellularly Without Relying on Type III Secretion Systems
Provision of Preferred Nutrients to Macrophages Enables Salmonella to Replicate Intracellularly Without Relying on Type III Secretion Systems
Garcia-Rodriguez, F.-J.; Valenzuela, C.; Bernal-Bayard, J.; Escoll, P.
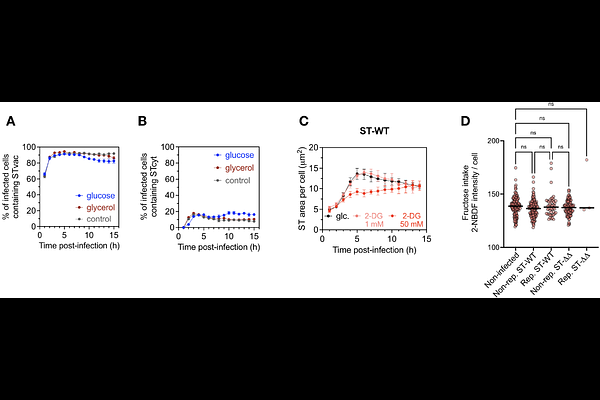
AbstractIntracellular survival and replication within macrophages are key virulence determinants of Salmonella enterica serovar Typhimurium. This phenomenon is traditionally attributed to the activity of its two Type III Secretion Systems (T3SS) and their associated effectors. A critical challenge for these bacteria is acquiring nutrients from inside the host cell. Thus, they modulate the metabolism of host cells to replicate. Given the metabolic plasticity of macrophages, a key unresolved question is how their metabolic heterogeneity shapes intracellular Salmonella replication. By using human primary macrophages and live-cell imaging to monitor bacterial dynamics at the single-cell level, we revealed that Salmonella does not replicate in all infected cells. However, supplementation with specific carbon sources used by Salmonella during infection accelerated bacterial replication and increased the proportion of macrophages showing replicative bacteria. Remarkably, this occurred even in the absence of functional T3SSs, as a {Delta}prgH/ssaV double mutant was able to replicate in a subset of infected cells under favorable nutrient conditions. These phenotypes are further amplified in macrophages with higher glycolytic activity, such as the murine RAW 264.7 cell line. Further analyses demonstrated that enhanced Salmonella replication is not strictly dependent on host glycolytic activity but is instead driven by the ability of the host cell to take up the nutrients Salmonella prefers for its replication early during infection. In summary, our findings suggest that the dependence of Salmonella on its T3SSs for intracellular replication can be bypassed when host cells provide optimal access to key nutrients and highlight the impact of metabolic heterogeneity in shaping intracellular bacterial replication during infection of macrophages.