mRNA Vaccine-Induced SARS-CoV-2 Spike-Specific IFN-γ and IL-2 T-cell Responses are Predictive of Serological Neutralization and are Transiently Enhanced by Pre-Existing Cross-Reactive Immunity
mRNA Vaccine-Induced SARS-CoV-2 Spike-Specific IFN-γ and IL-2 T-cell Responses are Predictive of Serological Neutralization and are Transiently Enhanced by Pre-Existing Cross-Reactive Immunity
Samaan, P.; Korosec, C.; Budylowski, P.; Chau, S. L. L.; Pasculescu, A.; Qi, F.; Delgado-Brand, M.; Tursun, T. R.; Mailhot, G.; Dayam, R. M.; Arnold, C. R.; Langlois, M.-A.; Patel, A.; de Launay, K. Q.; Boyd, J. M.; Takaoka, A.; Colwill, K.; McGeer, A.; Straus, S.; Gingras, A.-C.; Heffernen, J. M.; Ostrowski, M.
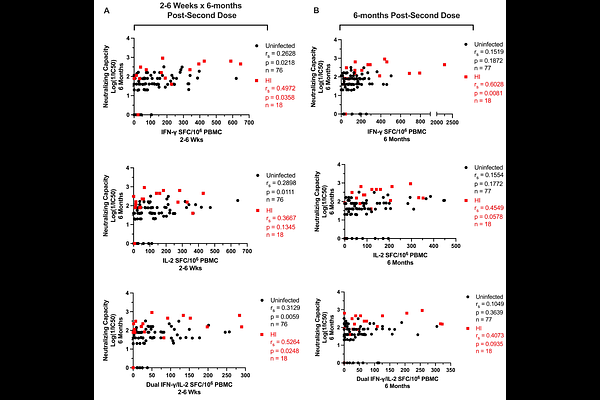
AbstractThe contributions of SARS-CoV-2-specific T-cells to vaccine efficacy and durability are unclear. We investigated the relationships between mRNA vaccine-induced spike-specific IFN-gamma and IL-2 T-cell responses, anti-spike/RBD IgG/IgA antibodies, and live virus neutralizing capacity in long-term-care-home staff doubly vaccinated with BNT162b2 or mRNA-1273. The impacts of pre-existing cross-reactive T-cell immunity to SARS-CoV-2 on cellular and humoral responses to vaccination were additionally assessed. Mathematical modelling of the kinetics of spike-specific IFN-gamma and IL-2 T-cell responses over 6-months post-second dose was bifurcated into recipients who exhibited gradual increases (54% and 42%, respectively) with doubling times of 173 days, or decreases (46% and 58%, respectively) with half-lives of 115 days. Differences in kinetics did not correlate with any clinical phenotypes, although increases were proposed to be due to subclinical viral exposures. Serological anti-spike/RBD IgG/IgA antibody levels had otherwise decayed in all participants with half-lives of 55, 53, 76, and 59 days, respectively. Spike-specific T-cell responses induced at 2-6 weeks correlated with live viral neutralization at 6-months post-second dose, especially in hybrid immune individuals. Participants with pre-existing cross-reactive T-cell immunity to SARS-CoV-2 exhibited greater spike-specific T-cell responses, reduced anti-RBD IgA antibody levels, and a trending increase in neutralization at 2-6 weeks post-second dose. Non-spike-specific T-cells predominantly targeted SARS-CoV-2 non-structural protein at 6-months post-second dose in cross-reactive participants. mRNA vaccination was lastly shown to induce off-target T-cell responses against unrelated antigens. In summary, vaccine-induced spike-specific T-cell immunity appeared to influence serological neutralizing capacity, with only a modest effect induced by pre-existing cross-reactivity.