A Continuously Oxygenated Macroencapsulation System Enables High-Density Packing and Delivery of Insulin-Secreting Cells
A Continuously Oxygenated Macroencapsulation System Enables High-Density Packing and Delivery of Insulin-Secreting Cells
Pham, T. T.; Tran, P. L.; Tempelman, L. A.; Stone, S. G.; Piccirillo, C.; Li, A.; Flanders, J. A.; Ma, M.
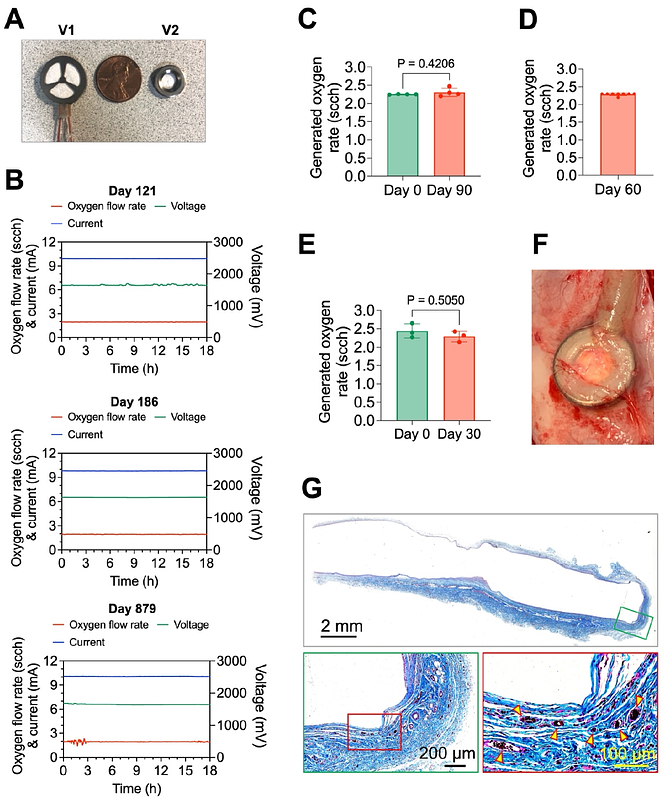
AbstractThe encapsulation of insulin-secreting cells within immuno-protective systems holds significant promise for curative treatment of type 1 diabetes without immunosuppression. A major challenge, however, remains the inadequate oxygen tension within the encapsulation systems, which compromises the survival and function of encapsulated cells and necessitates low packing density and impractically large systems to deliver a curative cell mass. In this study, we present a novel cell encapsulation system capable of generating oxygen via the electrolysis of tissue moisture to provide a continuous oxygen supply to densely packed insulin-secreting cells. Our system comprises a miniaturized implantable electrochemical oxygen generator (iEOG) and a scalable cylindrical cell encapsulation pouch, designed in a linear configuration to facilitate minimally invasive implantation and retrieval. The oxygen generation from the system was shown to be precisely controlled, stable, and capable of supporting clinically relevant doses of pancreatic islets. In vitro studies demonstrated that the oxygenated system effectively maintained the viability and function of insulinoma cell aggregates and human pancreatic islets at densities of 60,000 islet equivalents per mL (IEQ/mL) or 4,200 IEQ/cm2 under a hypoxic cell culture condition (1% oxygen). In an allogeneic rat model, the oxygenated systems containing pancreatic islets implanted into the poorly vascularized but clinically attractive subcutaneous space at a density of 60,000 IEQ/mL successfully reversed diabetes for up to about 3 months without the need for immunosuppression, while animals implanted with non-oxygenated systems remained diabetic. Most (~ 90%) of the pancreatic islets encapsulated in the continuously oxygenated systems were found viable and functional upon retrieval. These findings suggest the feasibility of using continuous oxygenation to support insulin-secreting cells at high loading densities in subcutaneous space, enabling the development of an encapsulation system with clinically practical dimensions.