Automated high-throughput patch clamp electrophysiology of hiPSC-derived neuronal models
Automated high-throughput patch clamp electrophysiology of hiPSC-derived neuronal models
Farinelli, F.; Ostlund, I.; Sripathy, S. R.; Das, D.; Shim, G.; Myung, S.; Straub, R. E.; Maher, B. J.
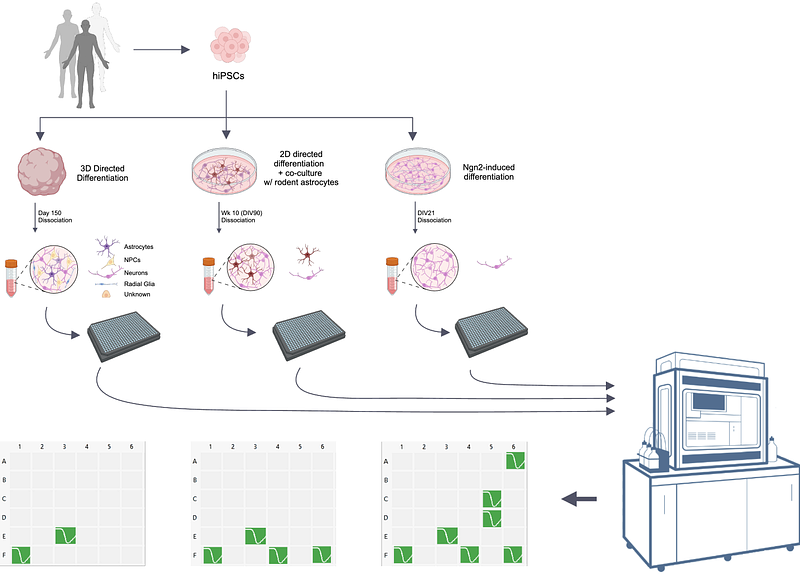
AbstractThe advent of human induced pluripotent stem cells (hiPSCs) and their differentiation into neurons and brain organoids has revolutionized our ability to model brain disorders in a human context. However, current technologies to assay the electrophysiological properties of human neurons in these models remain limited by throughput, as single-cell manual patch clamp is laborious and resource intensive. Here, we provide methods to perform automated high-throughput patch-clamp (APC) on hiPSC-derived neurons. We describe how to dissociate and perform voltage-clamp recordings on human neurons from three well-established protocols - 2D directed differentiation of cortical neurons, NGN2-induced neurons, and 3D cortical organoids - using the Nanion Syncropatch 384, a commercially available high-throughput APC system. Using this approach, we investigated the biophysical properties of voltage-gated sodium channels (VGSCs) and provide direct comparisons between manual and APC recordings across all three hiPSC-derived model systems. We demonstrate the capability of this automated system for pharmacological analysis of native human VGSC isoforms, which will enable compound screening approaches. Lastly, we provide methods to sort specific cellular populations within these hiPSC models using fluorescence-activated cell sorting (FACS) followed by APC. These methods and results provide a transformative and novel high-throughput technique for quantifying passive and active membrane properties in cell-type specific and/or genetically modified hiPSC-derived neurons.