Affinity-matured CD72-targeting Nanobody CAR T-cells Enhance Elimination of Antigen-Low B-cell Malignancies
Affinity-matured CD72-targeting Nanobody CAR T-cells Enhance Elimination of Antigen-Low B-cell Malignancies
Izgutdina, A.; Rashid, T.; Temple, W. C.; Patino-Escobar, B.; Walunj, S.; Geng, H.; Takamatsu, H.; Gil-Alos, D.; Kang, A. S.; Ramos, E.; Chen, S.-Y.; Johnson, H.; Nix, M. A.; Naik, A.; Yuan, C. M.; Wang, H.-W.; Aminov, S.; Sahu, S.; Larson, R. C.; Carpenter, C.; Salangsang, F.; Phojanakong, P.; Camara Serrano, J. A.; Tariq, I.; Zakraoui, O.; Steri, V.; Valeri, A.; Martinez-Lopez, J.; Maus, M. V.; Parekh, S.; Verma, A.; Shah, N. N.; Wiita, A.
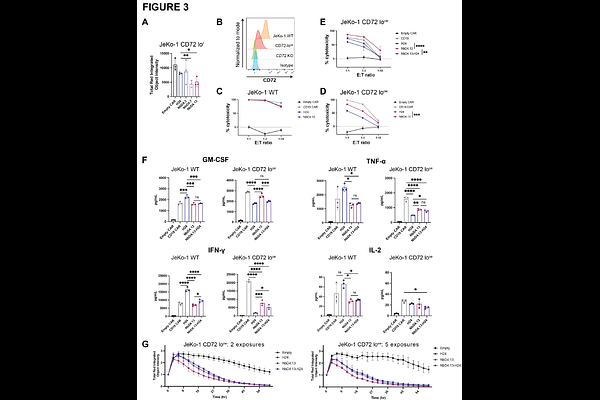
AbstractBackground: Chimeric antigen receptor (CAR) T-cell therapies are highly efficacious for several different hematologic cancers. However, for most CAR T targets it is observed that low surface antigen density on tumors can significantly reduce therapeutic efficacy. Here, we explore this dynamic in the context of CD72, a surface antigen we recently found as a promising target for refractory B-cell cancers, but for which CD72 low antigen density can lead to therapeutic resistance in preclinical models. Methods: Primary samples were accessed via institutional review board-approved protocols. Affinity-matured and humanized nanobody clones were previously described in Temple et al.1 CAR T-cells were generated via lentiviral transduction. In vitro cytotoxicity assays were performed using luciferase-labeled cell lines. In vivo studies were performed using cell line- or patient-derived xenografts implanted in NOD scid gamma (NSG) mice. Results: We first confirmed ubiquitous CD72 expression across a range of primary B-cell non-Hodgkin lymphomas. We further found that after resistance to CD19-directed therapies, across both B-cell acute lymphoblastic leukemia (B-ALL) models and primary tumor samples, surface CD72 expression was largely preserved while CD22 expression was significantly diminished. Affinity maturation of a nanobody targeting CD72, when incorporated into chimeric antigen receptor (CAR) T-cells, led to more effective elimination in vitro of isogenic models of CD72 low-expressing tumors. These results suggested that nanobody-based CAR T-cells (nanoCARs) may exhibit a similar relationship between binder affinity, antigen expression, and efficacy as previously demonstrated only for scFv-based CAR T-cells. Surprisingly, however, this significantly improved in vitro efficacy only translated to modest in vivo survival benefit. As a parallel strategy to enhance CAR T function, we found that the small molecule bryostatin could also significantly increase CD72 surface antigen density on B-cell malignancy models. Structural modeling and biochemical analysis identified critical residues improving CD72 antigen recognition of our lead affinity-matured nanobody. Conclusions: Together, these findings support affinity-matured CD72 nanoCARs as a potential immunotherapy product for CD19-refractory B-cell cancers. Our results also suggest that for B-ALL in particular, CD72 may be a preferable second-line immunotherapy target over CD22.