Cas9 RNP Physiochemical Analysis for Enhanced CRISPR-AuNP Assembly and Function
Cas9 RNP Physiochemical Analysis for Enhanced CRISPR-AuNP Assembly and Function
Lane, D. D.; Gottimukkala, K. S. V.; Cunningham, R.; Cassidy, M. E.; Jwa, Y.; Castelli, J. M. P.; Adair, J. E.
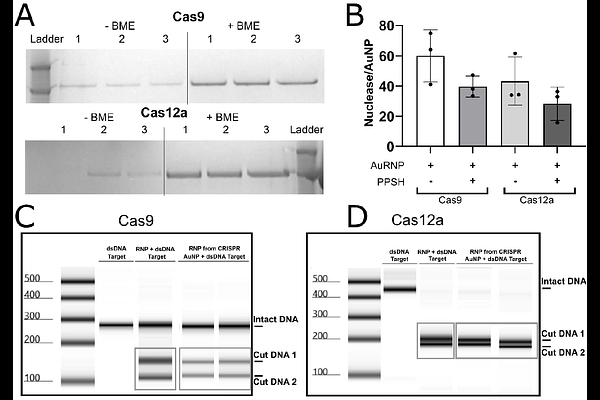
AbstractCRISPR therapy for hematological disease has proven effective for transplant dependent beta thalassemia and sickle cell anemia, with additional disease targets in sight. The success of these therapies relies on high rates of CRISPR-induced double strand DNA breaks ex vivo. To achieve these levels, CRISPR complexes are typically delivered by electroporation which is toxic to target hematopoietic stem and progenitor cells (HSPC). HSPC are then cultured in stimulating conditions that promote error-prone DNA repair, requiring conditioning with chemotherapy to facilitate engraftment after reinfusion. In vivo delivery by nanocarriers of CRISPR gene tools has the potential to mitigate this complexity and toxicity and make this revolutionary therapy globally available. To achieve in vivo delivery, the inherent restriction factors against oligonucleotide delivery to HSPCs that made ex vivo manipulation including electroporation and stimulation essential must be overcome. To this end, our group developed a CRISPR carrying gold nanoparticle (CRISPR-AuNP) capable of delivering either Cas9 or Cas12a CRISPRs as ribonucleoprotein complexes (RNP) without compromising HSPC fitness. However, the most commonly used CRISPR, Cas9, demonstrated inconsistent activity in this delivery system, with lower than expected activity relative to Cas12a, and no observable activity in humanized mice in vivo. Investigation of Cas9 RNP biophysics relative to Cas12a revealed duplex RNA instability during the initial loading onto Au cores, resulting in undetectable Cas9 loading to the particle surface. Here we demonstrate preformation of RNP before loading, coupled with optimization of the loading chemistry and conditions resulted in 39.6 {+/-} 7.0 Cas9 RNP/AuNP without compromising RNP activity in both in vitro assays and in primary human HSPC. The same alterations improved Cas12a RNP/AuNP loading 10-fold over previously reported levels. To achieve particle stability, the reported polyethyleneimine outer coating was altered to include PEGylation and the resulting 2nd generation CRISPR-AuNP demonstrates favorable nanoformulation characteristics for in vivo administration, with a hydrophilic, more neutral nanoparticle surface. Direct treatment of HSPC in vitro showed 72.5 {+/-} 7.37% 2nd generation CRISPR-AuNP positive primary human HSPC by confocal tracking, but with endosomal accumulation and low rates of gene editing consistent with low levels of endosomal escape.