Fluorescein Angiography Image-AI Based Early Diabetic Retinopathy Detection
Fluorescein Angiography Image-AI Based Early Diabetic Retinopathy Detection
Peng, Y.; Toh, H.; Jiang, P.
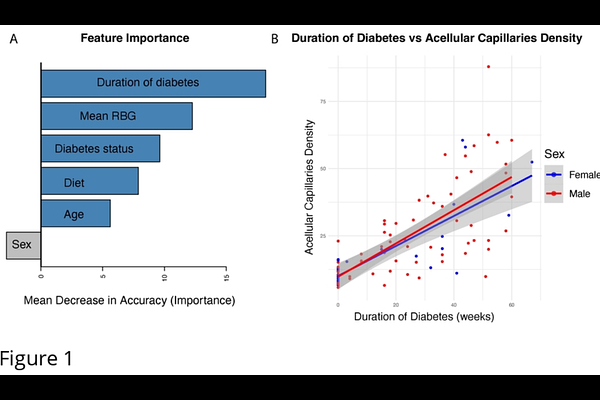
AbstractFluorescein angiography (FA) is widely regarded as the clinical gold standard for diagnosing diabetic retinopathy (DR), based on a range of observable symptoms, including microaneurysms, retinal hemorrhages, and vascular leakage. However, early detection of DR remains challenging, as these symptoms may not manifest simultaneously and are often too subtle to detect. Acellular capillary density is currently the only quantitative metric used to assess the risk and severity of DR, particularly in its early stages when these clinical symptoms are minimal or absent. Unfortunately, measuring acellular capillary density to detect pre-symptomatic diabetic retinopathy (DR) requires destructive techniques such as trypsin digest to remove non-vascular tissues, making it unsuitable for human patients, while current imaging techniques such as FA does not have resolution to quantify acellular capillary density, limiting the utility of non-invasive approaches for early detection of DR. In this study, we used Nile rat as a DR model to train an AI deep learning model based on FA images to detect retinas with acellular capillary density [≥]18 counts per mm2, which is considered as pre-symptomatic (before clinically observed DR symptoms) DR. Our model achieves an accuracy of 80.85%, with a sensitivity of 84.21%, specificity of 75.68%, and an area under the receiver operating characteristic (ROC) curve (AUC) of 0.86. We also showed that integrating the duration of diabetes as a pre-knowledge into a Bayesian framework with real time AI-image prediction can enhance the prediction power with AUC of ROC of 0.9. Using the first sequenced Nile rat genome and our RNA-seq data, we identified 43 gene markers that signified a shift from a normal state to a transitional phase preceding early diabetic retinopathy (DR). While PDGF-B/PDGFR{beta} signaling is typically associated with pericyte loss and increased acellular capillary density, which are hallmarks of early DR, none of the identified markers were related to this pathway. Instead, three genes (Bcl2a1, Birc5, and Il20rb) out of these 43 biomarkers are associated with inflammation or response to inflammation suggesting that inflammation may contribute to the shift in acellular capillary density from <16 (normal) to 17-18 counts (transitional phase preceding early DR) per mm2 before pericyte loss occurs, providing new insights into the early pathogenesis of DR.