Bacterial glycosphingolipids orchestrate colonization and immune modulation in neonatal host
Bacterial glycosphingolipids orchestrate colonization and immune modulation in neonatal host
Heo, K.; Jung, D.-J.; Yoo, J.-S.; Goh, B.; Kasper, D. L.; Oh, S. F.
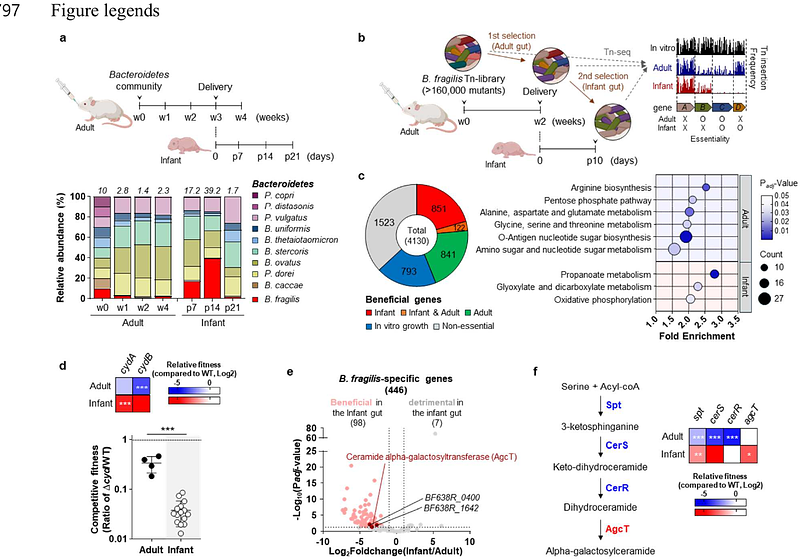
AbstractSymbiotic microbiota has co-existed with the mammalian host over millennia, conserving a stable community structure generation after generation. During the vertical transmission, gut symbionts rapidly colonize the unoccupied host lumen, nonetheless, how symbionts adapt to the dynamic changes of host environment, and contribute to the structural and immunological maturation remains elusive. Here, we show that the early gut symbiont Bacteroides fragilis produces a species- and stage-specific sphingolipid, alpha-galactosylceramide (BfaGC), that orchestrates neonatal colonization and immune modulation. BfaGC stabilizes membrane integrity and facilitates aerobic respiration, providing a critical advantage under early-life oxygen exposure. Temporally induced in the neonatal gut, BfaGC is necessary to regulate colonic type I natural killer T (NKT) cells, highlighting metabolic adaptation of the symbiont is synchronized with the time-sensitive host development. These findings reveal a mutualistic benefit exerted by endobiotic lipid metabolites in host-microbe interactions and provide new insights into species-specific mechanisms in early microbiota establishment and host immune education.