Antigen-specific Th17 T cells offset the age-related decline in durable T cell immunity
Antigen-specific Th17 T cells offset the age-related decline in durable T cell immunity
Sturmlechner, I.; Jain, A.; Jiang, J.; Okuyama, H.; Mu, Y.; Own, M.; Weyand, C.; Goronzy, J.
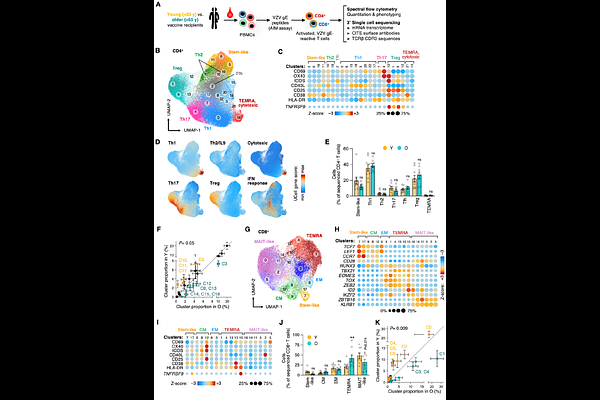
AbstractOlder adults are susceptible to infections, in part due to waning of immune memory. To determine mechanisms that determine long-lasting versus short-term immunity, we examined varicella zoster virus (VZV) vaccination as a model system. We contrasted VZV antigen-specific T cells several years after vaccination in adults who had been vaccinated at young (<20 years) or older age (>50 years) with a live-attenuated vaccine that confers durable protection only when given at young age, or with an adjuvanted VZV component vaccine that elicits effective, long-lasting immunity in older adults. CD8+ T cells were highly sensitive to age-related changes showing T cell subset shifts, loss in TCR diversity and reduced stem-like features while gaining NK-like signatures without evidence for cellular senescence or exhaustion. VZV-specific CD4+ T cells were largely resilient to age and maintained phenotypic and TCR diversity. Immunization of older adults with the adjuvanted VZV vaccine did not reverse age-associated defects in CD8+ T cells. Instead, it selectively improved the functionality of VZV-specific Th17 CD4+ T cells and prevented their acquisition of Treg features, likely as consequence of lipid metabolic pathways. Collectively, our data indicate that effective vaccination in older adults is supported by the generation of a durable, antigen-specific CD4+ Th17 population that resists mis-differentiation into Tregs and that compensates for age-related defects in CD8+ T cells.