Induced Proteinuria Enhances Adeno-Associated Virus Transduction of Renal Tubule Epithelial Cells After Intravenous Administration
Induced Proteinuria Enhances Adeno-Associated Virus Transduction of Renal Tubule Epithelial Cells After Intravenous Administration
Rubin, J. D.; Ayasoufi, K.; McGlinch, E. B.; Hansen, M. J.; Harris, P. C.; Torres, V. E.; Barry, M. A.
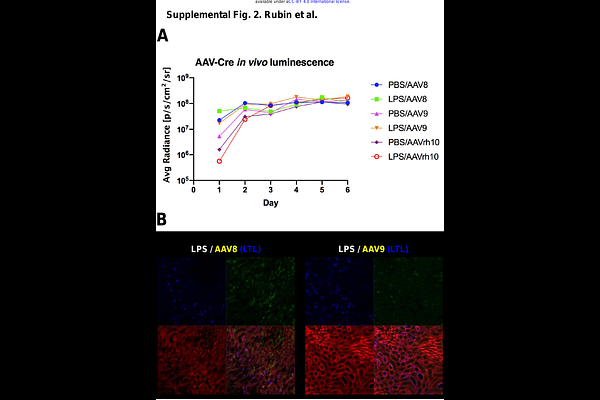
AbstractA variety of genetic diseases of the kidney tubule are amenable to correction via gene therapy. However, gene delivery to renal tubule epithelial cells mediated by viral vectors via the blood is inefficient due to the permselectivity of the glomerular barrier. We hypothesized that effacement of podocyte foot processes would disrupt typical glomerular limitations on filtration and make renal tubule epithelial cells susceptible to transduction from viral vectors delivered intravenously. We determined that adeno-associated virus serotype 8 (AAV8) transduced significantly more epithelial cells in the kidney under the conditions of LPS-induced proteinuria. Use of AAV1 in tandem with LPS-induced proteinuria yielded an ideal two-pronged effect of both partially detargeting the liver and transducing the kidney with a higher bioluminescent signal than AAV8 did, and at half of the dose. Using adenovirus serotype 5 (Ad5) in conjunction with LPS-induced proteinuria showed that kidney transduction was enhanced, but only in glomerular cells. These studies mechanistically test the efficacy of different viral vectors and demonstrate their capacity to transduce kidney epithelial cells. This is a fundamental step in designing future treatments for kidney gene therapy.