Chlamydia muridarum Infection Impacts Murine Models of Intestinal Inflammation and Cancer
Chlamydia muridarum Infection Impacts Murine Models of Intestinal Inflammation and Cancer
Leung, G.; Carrasco, S. E.; Mourino, A. J.; Aydin, M.; Mammone, R.; Sonnenberg, G. F.; Diehl, G.; Artis, D.; Yano, H.; Arbona, R. J. R.; Lipman, N. S.
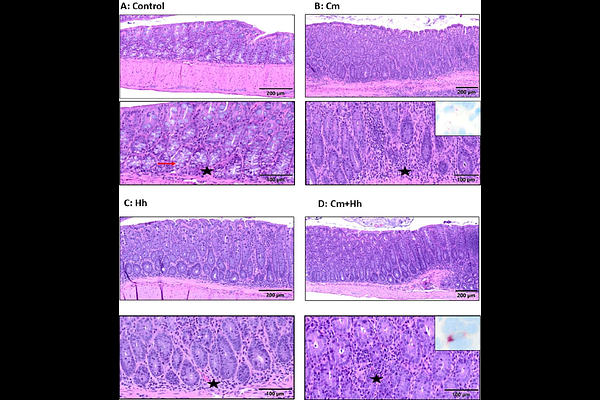
AbstractChlamydia muridarum (Cm) has reemerged as a prevalent infectious agent in research mouse colonies. Despite its prevalence and ability to persistently colonize the murine gastrointestinal tract, few studies have evaluated the potential impact of Cm on experimental models of gastrointestinal disease. Studies were conducted to evaluate the impact of Cm on the Citrobacter rodentium (Cr), Trichuris muris (Tm) and Il10-/- mouse models of intestinal inflammation, as well as on tumorigenesis in the ApcMin/+ mouse following administration of DSS. Naive C57BL/6J (B6), B6.129P2-Il10tm1Cgn/J (Il10-/-) and C57BL/6J-ApcMin/J (ApcMin/+) mice were infected with Cm by cohousing with chronic Cm-shedding BALB/cJ mice for 2 weeks; controls were cohoused with Cm-free mice. After cohousing, B6 mice (n=8 Cm-infected and free) were infected with Citrobacter rodentium (Cr; 109 CFU orally) or Trichuris muris (Tm; 200 ova orally). Il10-/- mice (n=8/group with and without Helicobacter hepaticus [108 CFU/mouse] and with and without Cm) and ApcMin/+ mice (n=8/group) received 2% DSS for 7 days in drinking water after cohousing. Mice were sacrificed 14-days post-Cr infection, 18-days post-Tm infection, 70-days after cessation of cohousing with Il10-/- mice, and 28-days post-DSS administration to ApcMin/+ mice. The severity of the cecal and colonic lesions were evaluated and graded using a tiered, semi-quantitative scoring system. Cm infection attenuated colitis associated with Cr (p=0.03), had no effect on Tm associated pathology (p=0.22), worsened colitis in Il10-/- mice in the absence of H. hepaticus (p=0.007), and reduced chemically induced colonic tumorigenesis in ApcMin/+ mice (p=0.004). Thus, Cm colonization differentially impacts several models of intestinal inflammation and tumorigenesis, and the presence of this bacterium in mouse colonies should be considered as a variable in these experimental readouts.