Sp1 mechanotransduction regulates breast cancer cell invasion in response to multiple tumor-mimicking extracellular matrix cues
Sp1 mechanotransduction regulates breast cancer cell invasion in response to multiple tumor-mimicking extracellular matrix cues
Sharma, A.; Steger, R. F.; Li, J. M.; Baude, J. A.; Heom, K. A.; Dey, S. S.; Stowers, R. S.
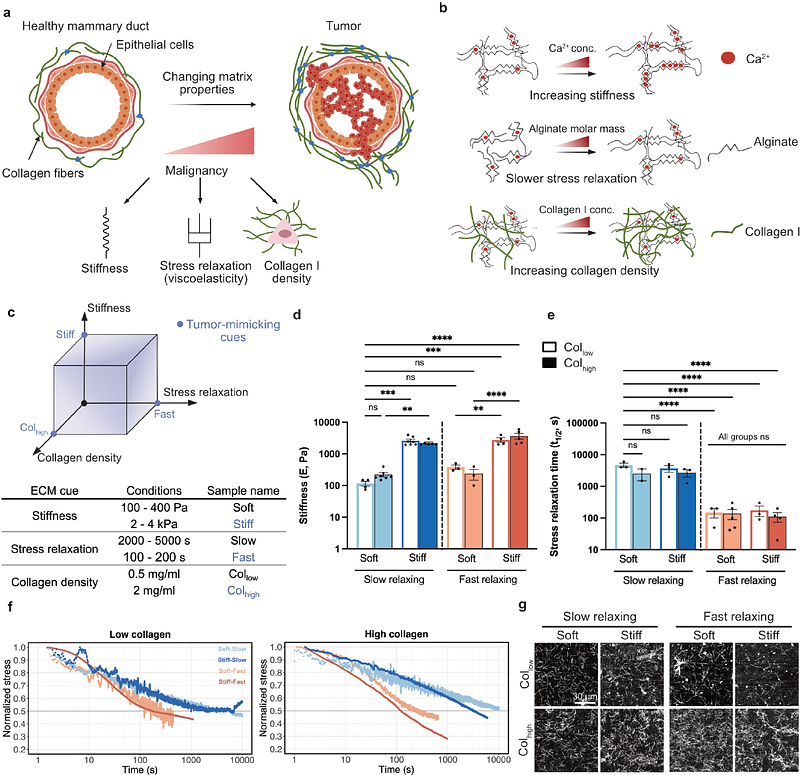
AbstractBreast cancer progression is marked by extracellular matrix (ECM) remodeling, including increased stiffness, faster stress relaxation, and elevated collagen levels. In vitro experiments have revealed a role for each of these factors to individually promote malignant behavior, but their combined effects remain unclear. To address this, we developed alginate-collagen hydrogels with independently tunable stiffness, stress relaxation, and collagen density. We show that these combined tumor-mimicking ECM cues reinforced invasive morphologies and promoted spheroid invasion in breast cancer and mammary epithelial cells. High stiffness and low collagen density in slow-relaxing matrices led to the greatest cell migration speed and displacement. RNA-seq revealed Sp1 target gene enrichment in response to both individual and combined ECM cues, with a greater enrichment observed under multiple cues. Notably, high expression of Sp1 target genes upregulated by fast stress relaxation correlated with poor patient survival. Mechanistically, we found that phosphorylated-Sp1 (T453) was increasingly located in the nucleus in stiff and/or fast relaxing matrices, which was regulated by PI3K and ERK1/2 signaling, as well as actomyosin contractility. This study emphasizes how multiple ECM cues in complex microenvironments reinforce malignant traits and supports an emerging role for Sp1 as a mechanoresponsive transcription factor.