Norbornene homopolymerization limits cell spreading in thiol-ene photoclick hydrogels
Norbornene homopolymerization limits cell spreading in thiol-ene photoclick hydrogels
Gentry, J. L.; Caliari, S. R.
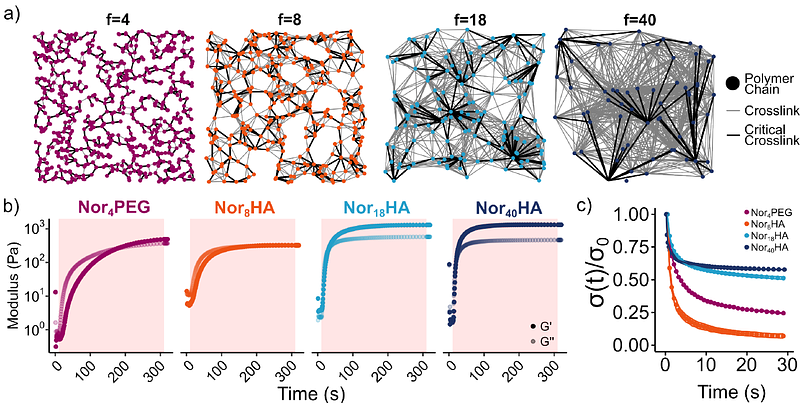
AbstractThiol-ene click chemistry is a powerful tool for designing hydrogels mimicking the mechanical and biochemical properties of 3D cellular microenvironments. The high selectivity of thiol-norbornene step-growth polymerization enables precise control of crosslinking mechanism, circumventing the alkene homopolymerization present in other systems that can prevent encapsulated cell spreading. Limited stress relaxation of a dynamically-crosslinked norbornene-modified hyaluronic acid (NorHA) hydrogel employing a thiol-norbornene photoclick reaction led us to investigate the prevalence of norbornene homopolymerization in this supposed click reaction. Norbornene conversion was quantified in multiple thiol plus norbornene-modified polymer system permutations, revealing higher norbornene conversion than expected for 1:1 thiol-ene addition. We showed that decreasing the number of norbornenes per NorHA chain (f) mitigated network formation via norbornene homopolymerization. Dynamic hydrogels fabricated with NorHA of f = 8 (Nor8HA) exhibited 93.0 {+/-} 1.6% relaxation, while those fabricated with NorHA of f = 40 (Nor40HA) achieved only 42.3 {+/-} 0.1% relaxation. As early as day 3 of culture, Nor8HA hydrogels facilitated spreading of encapsulated human mesenchymal stromal cells (hMSCs) into a spindle-like morphology (aspect ratio: 2.95 {+/-} 0.38), while Nor40HA hydrogels appeared to constrain cells into a spherical or compact star morphology (aspect ratio: 1.22 {+/-} 0.01). Inference of a single-cell morphological space derived from a shape-matching distance metric validated the two distinct hMSC morphological phenotypes primarily associated with polymer f. Despite its widespread use as a click reaction, radical-mediated thiol-norbornene crosslinking was found to not be stoichiometric in dilute aqueous conditions used to fabricate hydrogels. Altering network topology through polymer f enabled the rescue of hydrogel dynamic behavior and encapsulated hMSC spreading, despite the presence of norbornene homopolymerization, highlighting the need to consider network-level properties when designing engineered cellular microenvironments.