CD4+ T cells promote fibrosis during metabolic dysfunction-associated steatohepatitis
CD4+ T cells promote fibrosis during metabolic dysfunction-associated steatohepatitis
Valenzuela-Perez, L.; Kim Lee, H. S.; Bayer, R. L.; Mishra, S. K.; Washington, A. M.; Guo, Q.; Herman, A.; Graham, R. P.; Sidahmed, M. M.; Ssali, E.; Hassan, A. A.; Dinc, E. J.; Pavelko, K. D.; Gores, G. J.; Starlinger, P.; Revelo, X. S.; Ibrahim, S. H.; Kostallari, E.; Bamidele, A. O.; Hirsova, P.
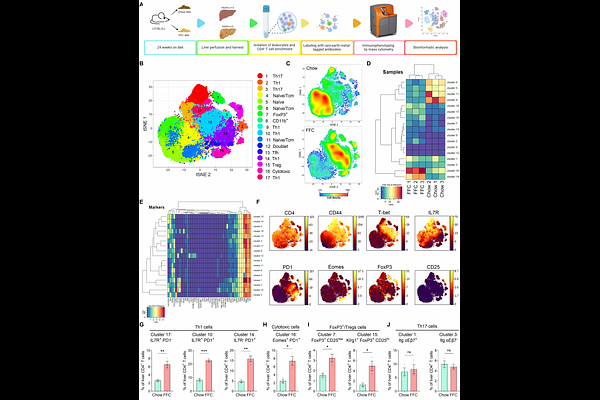
AbstractUnresolved inflammation and fibrosis are the two key features of metabolic dysfunction-associated steatohepatitis (MASH), a progressive form of steatotic liver disease that can evolve into cirrhosis and liver cancer. Although innate immunity has been well studied in MASH, the role of CD4+ T cells remains underexplored despite their potential to coordinate immune responses by providing help to other immune cells, promoting inflammation, or regulating immune activity through effector and regulatory subsets. To better understand the role of CD4+ T cells in the pathogenesis of MASH, we comprehensively characterized hepatic CD4+ T cells in murine and human MASH at a single-cell protein, transcriptional, and functional level. Mass cytometry and CITE-sequencing revealed a marked shift in intrahepatic CD4+ T-cell composition in MASH, with enrichment of Th1, regulatory, and cytotoxic CD4+ T cells. Similar phenotypic changes were mirrored in the peripheral blood and validated in human MASH samples. Functional assays demonstrated increased production of IFN{gamma} and TNF by hepatic CD4+ T cells, highlighting their proinflammatory effector activity. Transcriptomic profiling identified Tnfrsf4 (OX40) upregulation in hepatic CD4+ T cells during MASH. Therapeutic blockade of the OX40L-OX40 axis reversed hepatic fibrosis and improved histologic disease scores in mice with established MASH, and also decreased inflammatory markers in a human ex vivo liver model. Together, these studies provide a proteogenomic single-cell atlas for hepatic CD4+ T cells and uncover a CD4+ T cell-dependent immunopathogenic circuit as a promising immunotherapeutic target to alleviate MASH and liver fibrosis.