Multi-Tissue Profiling Reveals tissue-specific protein regulation and relationships Between Protein Quantitative Trait Loci (pQTLs) and Cardiometabolic Disease
Multi-Tissue Profiling Reveals tissue-specific protein regulation and relationships Between Protein Quantitative Trait Loci (pQTLs) and Cardiometabolic Disease
Hartley, A. E.; Sukhavasi, K.; Hu, S.; Traylor, M.; Gonzalez-Ramirez, M.; Hanghoj, K. E.; Talukdar, H.; Ruusalepp, A.; Bjorkegren, E.; Bjorkegren, J.; Howson, J. M.; Jamshidi, Y.
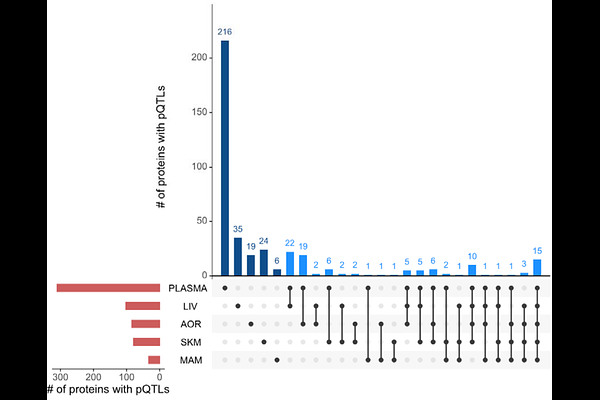
AbstractIntegrating genetic data with protein levels, known as protein quantitative trait loci (pQTLs), can enhance our understanding of disease mechanisms and provide actionable insights for drug discovery, by guiding the direction of therapeutic interventions, clarifying mechanisms of action, and predicting potential side effects. However, most pQTL studies have focused on the plasma proteome, overlooking tissue-specific effects. Here, we investigate the plasma and tissue proteome and derive tissue-specific pQTLs in a unique dataset derived from a cohort of 284 STARNET patients, predominantly male, with a mean age of 65 years and a high prevalence of coronary artery disease (CAD).Importantly, our dataset includes paired tissue samples from aortic wall, mammary artery, liver, and skeletal muscle alongside plasma, allowing for a comprehensive comparative analysis across tissues- all from the same individuals. We employed the Olink Explore 3.2k platform to assess relative protein levels in each tissue. We identify 608 cis-pQTLs, the majority of which are found in plasma, reflecting greater protein variability. Notably, we find 13 proteins with exclusive tissue-specific pQTLs, underscoring distinct as well as shared genetic influences across tissues. Colocalization analyses reveal shared genetic regulation between tissue proteins and cardiometabolic traits, including LDL, HDL, and triglycerides levels, implicating proteins such as PNLIPRP2, SORT1, and PRSS53 as potential mediators of lipid regulation. Furthermore, Mendelian randomization analyses suggest a liver-specific role for SORT1 and PSRC1 in modulating CAD risk and lipid profiles.