Modulation of the GT Family 47 clade B gene affects arabinan deposition in elaters of Marchantia polymorpha
Modulation of the GT Family 47 clade B gene affects arabinan deposition in elaters of Marchantia polymorpha
Kang, H. S. F.; Lampugnani, E. R.; Tong, X.; Prabhakar, P. K.; Flores-Sandoval, E.; Hansen, J.; Jorgensen, B.; Bowman, J. L.; Urbanowicz, B. R.; Ebert, B.; Persson, S.
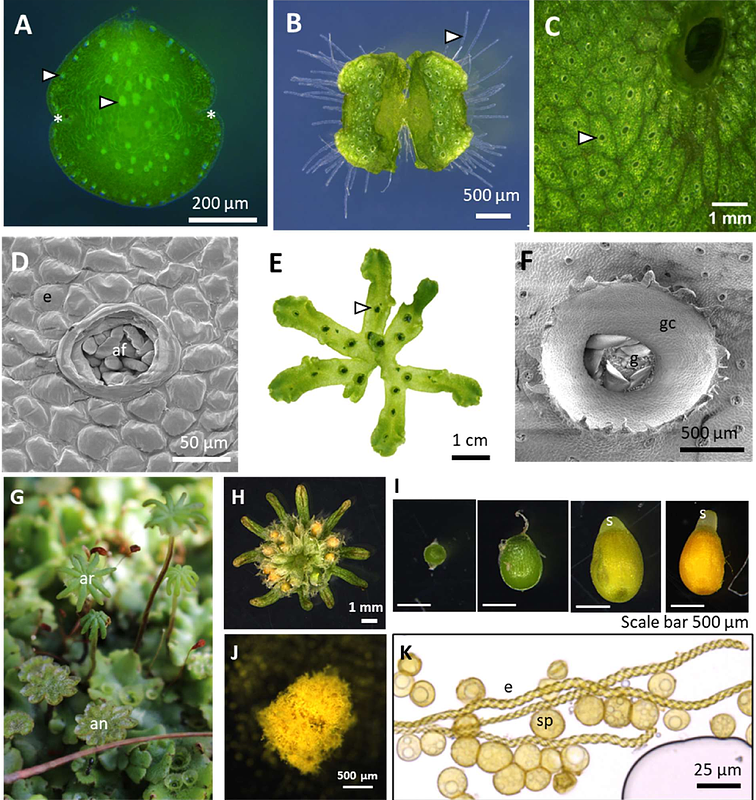
AbstractThe plant cell wall polymer -1,5-linked arabinan is associated with important functions in plant physiology, such as cell wall flexibility and desiccation tolerance in angiosperms. However, its biosynthetic mechanism remains obscure. A putative arabinan arabinosyltransferase (AraT) is the Arabidopsis ARABINAN-DEFICIENT1 in GT family 47 clade B (GT47B), but its role is inferred by its mutant cell wall phenotypes, and not through in vitro assays. With seven other genes in the clade, investigating the function of the members in GT47B is hampered by the high genetic redundancy in Arabidopsis. Instead, we probe the function of the only two genes in GT47B in the liverwort model organism Marchantia polymorpha, named MpARAD-Like 1 (MpARADL1) and MpARADL2. Mparadl1 and Mparadl2 loss-of-function mutants and overexpression lines were generated, and their cell wall composition was probed using Comprehensive Microarray Polymer Profiling (CoMPP), glycosyl linkage analysis and immunolabelling. Interestingly, Mparadl2 mutants have much less of the 1,5--L-arabinan epitope in elaters since LM6 antibody recognition showed a notable reduction. However, 5-linked Araf levels in the Marchantia thallus tested with glycosyl linkage analysis are comparable between mutants and wild type, suggesting that there is another enzyme forming 5-Araf linkages in Marchantia. Our attempts to obtain the biochemical activity of these enzymes through the expression and purification of AtARAD1, MpARADL1 and MpARADL2 proteins in the HEK293 cell heterologous expression system were unsuccessful. Therefore, we suspect that an evolutionarily conserved, and clade-specific mechanism is required to confer solubility for purification and subsequent activity characterisation of GT47B proteins. Previous studies have shown that co-expression of protein interaction partners can enhance protein solubility in the HEK293 cell system, but co-expression of MpARADL2 with AtARAD1 and MpARADL1 did not yield soluble proteins, prompting further investigation into the protein-protein interactors of GT47B proteins.