Fibroblasts regulate lymphatic barrier functions in a tissue dependent manner
Fibroblasts regulate lymphatic barrier functions in a tissue dependent manner
Ejazi, S. A.; Abdulkarimu, A.; Hsiao, T.; Garcia-Vivas, R. D.; Maisel, K.
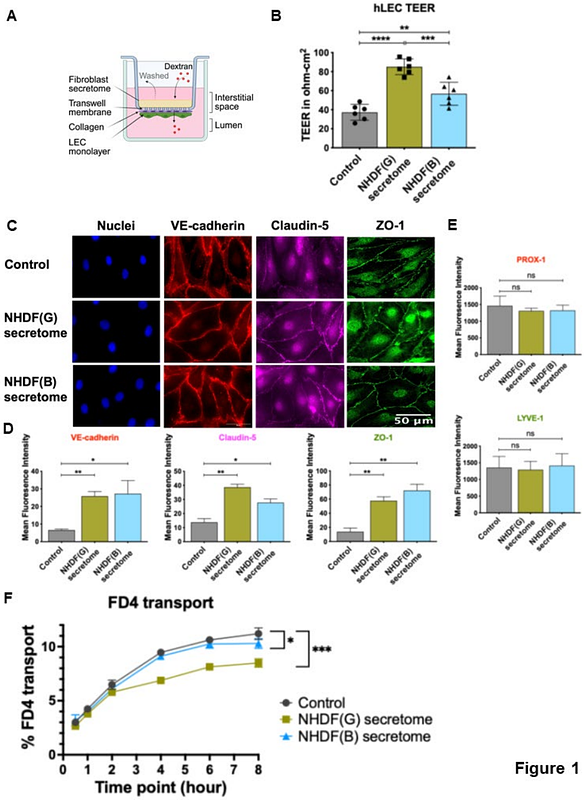
AbstractThe lymphatic vasculature plays a vital role in clearing interstitial fluid and antigens from tissues and guiding immune cell migration. Junctions between lymphatic endothelial cells (LECs) are important for preserving vascular integrity and permitting transport or migration into the vessel. Tight junction proteins, like ZO-1, work alongside adherens junction proteins, like VE-cadherin, to regulate lymphatic barrier function and permeability. Fibroblasts contribute to the lymphatic interstitial environment by producing extracellular matrix and producing growth factors that induce lymphangiogenesis. Despite their known roles, the direct impact of fibroblasts on LEC barrier functions, specifically permeability and cell-cell junctions, as well as the underlying mechanisms remains poorly understood. Here, we established a non-contact in vitro co-culture system to investigate how fibroblasts affect LEC junctions and permeability. We determined the effect of secreted factors (secretomes) from normal human dermal fibroblasts (NHDFs) on human LECs (hLECs) and found that these led to a >120% increase in the transendothelial electrical resistance (TEER) of hLEC monolayer. Immunofluorescence analysis revealed a >4-fold increase in the expression of ZO-1 and VE-cadherin, and a 24% reduction in transport of FITC- dextran upon NHDF secretome treatment. Co-culture of hLECs with NHDFs also more than doubled TEER, and increased ZO-1 and VE-cadherin expression by 1.3-1.6 and 2-fold, respectively, compared to untreated controls. Dextran permeability dropped by 15-30% after NHDF treatment. Finally, we found a significant increase in the continuity of ZO-1 junctions in hLECs co-cultured with NHDFs. Surprisingly, we found an opposite effects on hLEC junctional integrity and permeability with normal human lung fibroblast (NHLF) secretomes: NHLFs significantly lowered TEER along with ZO-1 expression and doubled dextran permeability across hLECs. Finally, we found that treatment of hLECs with thrombin, a potent inflammatory mediator, led to impaired cell junctions and increased transport only in the presence of NHDFs. This study highlights the role of fibroblasts in regulating lymphatic barrier function by modulating endothelial cell-cell junctions. Notably, their impact on LECs varies based on the tissue origin, revealing functional heterogeneity. Our findings offer valuable insights and novel therapeutic targets for modulating lymphatic functions in disease and provide foundations for strategies to improve lymphatic targeted drug delivery.