NewroBus for the brain: humanized TfR1-targeting nanobodies with high BBB permeability and cargo transport capacity
NewroBus for the brain: humanized TfR1-targeting nanobodies with high BBB permeability and cargo transport capacity
Yin, T.; Yesiltepe, M.; Metkar, S.; Ramon, A. E.; Greening, M.; Sormanni, P.; D'Adamio, L.
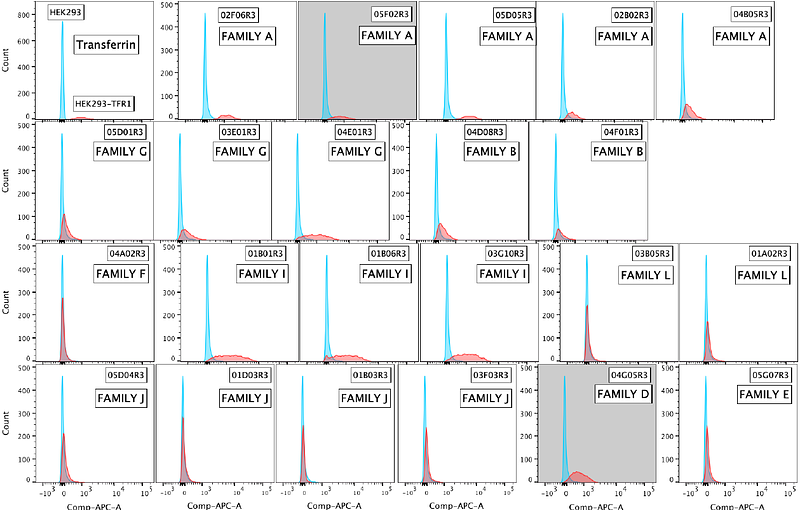
AbstractDelivery of therapeutics to the brain is restricted by the blood-brain barrier (BBB). A promising strategy to overcome this involves exploiting receptor-mediated transcytosis pathways, such as those mediated by transferrin receptor 1 (TfR1), which is highly expressed on brain endothelial cells and naturally transports iron-bound transferrin across the BBB. To leverage this mechanism, we immunized camelids with human TfR1 and cloned 470 VHH nanobody sequences from their B cells. From this repertoire, 24 nanobodies (TfR1b-Nbs) were identified that bind cell membrane human TfR1. These nanobodies were produced in CHO-S cells, a mammalian expression system widely used in therapeutic protein production for its ability to support proper folding, disulfide bond formation, and human-compatible post-translational modifications. CHO-S cells also generate very low endotoxin levels, making them suitable for clinical-grade manufacturing. TfR1b-Nbs were screened for binding to human TfR1, lack of interference with transferrin binding and TfR1-mediated iron uptake, and the ability to cross the BBB via human TfR1-mediated transcytosis in newly generated humanized Tfr1h knock-in rats. To improve developability and reduce potential immunogenicity, selected TfR1b-Nbs were humanized and optimized using the AbNatiV algorithm, enhancing humanness, solubility, and VHH-nativeness. Eight optimized TfR1b-Nbs retained BBB permeability and were fused to humanized TNF inhibitors (TNFI- or TNFI-{beta}), generating 16 heterodimers. Fusion to TNFI served as a functional readout, confirming that TfR1b-Nbs can shuttle biologically active, BBB-impermeable payloads into the CNS. All heterodimers demonstrated CNS delivery after intravenous administration, and selected constructs also reached the brain via subcutaneous injection, maintaining high serum and CSF levels for up to 72 hours. A pilot study with one heterodimer showed that chronic administration in rats humanized for both transferrin and TfR1 caused no hematological toxicity or signs of anemia, a key safety concern when targeting TfR1. These results establish humanized TfR1b-Nbs, designated NewroBus, as promising BBB shuttles for the safe and effective therapeutic delivery of biologics to the brain.