A Membrane-Disruptive Action of VBIT-4 Challenges Its Role as a Widely Used VDAC Oligomerization Inhibitor
A Membrane-Disruptive Action of VBIT-4 Challenges Its Role as a Widely Used VDAC Oligomerization Inhibitor
Ravishankar, V.; Borges-Araujo, L.; Lafargue, E.; Byrne, D.; Buzhinsky, N.; Wolfe, M.; Bautista, N.; Beyene, B.; Larimi, M.; Duneau, J.-P.; Sturgis, J.; Rajendran, M.; Bezrukov, S.; Casuso, I.; Rostovtseva, T. K.; Bergdoll, L.
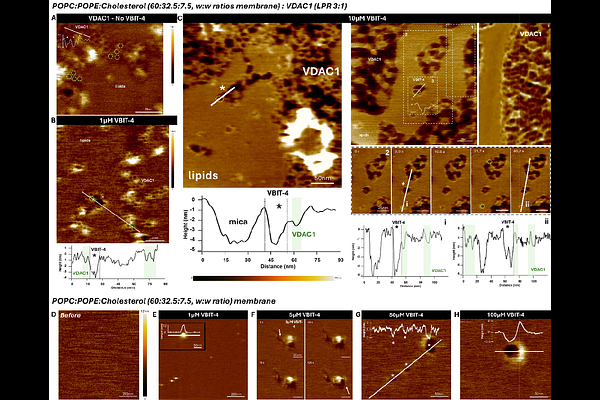
AbstractVDAC, the most abundant protein in the outer mitochondrial membrane, plays a central role in mitochondrial physiology. Its oligomerization has been contemplated to be involved in critical processes such as mtDNA release and apoptosis, yet the underlying molecular mechanisms remain poorly defined. VBIT-4, a small molecule widely used as a VDAC1 oligomerization inhibitor, has seen extensive applications over the past five years without proper mechanistic characterization. Using high-speed atomic force microscopy, we directly visualized VDAC1 oligomerization in planar lipid membranes and examined the effects of VBIT-4. Unexpectedly, VBIT-4 partitioned into lipid bilayers at micromolar concentrations and disrupted membrane structure even in the absence of VDAC1. Complementary approaches, including single-channel electrophysiology, microscale thermophoresis, and coarse-grained molecular dynamics, confirmed the membrane partitioning and destabilizing effects of VBIT-4. The compound also induced VDAC1-independent cytotoxicity in HeLa cells at concentrations above 10 microM. Our findings demonstrate that VBIT-4 disrupts membrane integrity by partitioning into lipids and inducing membrane defects rather than specifically inhibiting VDAC1 oligomerization, highlighting the need for caution when interpreting results and the importance of revisiting conclusions drawn from its prior use.