Molecular dynamics simulations reveal the impact of Ser295 phosphorylation on the structure of pyrin domain-containing NOD-like receptor 3
Molecular dynamics simulations reveal the impact of Ser295 phosphorylation on the structure of pyrin domain-containing NOD-like receptor 3
Sandall, C. F.; MacDonald, J. A.
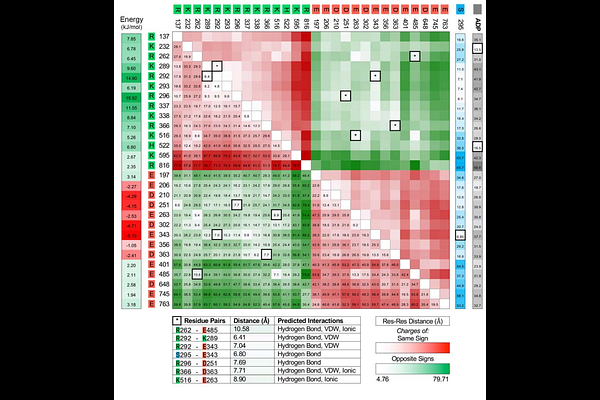
AbstractThe nucleotide-binding, leucine rich repeat, and pyrin-containing 3 (NLRP3) protein is regulated by phosphorylation of Ser295 in the NACHT domain. This post-translational modification is known to inhibit the enzymatic ATPase activity of NLRP3 and impede inflammasome complex assembly. In this study, modeled structures of unphosphorylated and pSer295-phosphorylated NLRP3-{Delta}PYD were subjected to molecular dynamics simulations. The outputs showed Ser295 phosphorylation to induce topologic distention of subdomains that comprise the NACHT domain. The relative orientation of important residues within the nucleotide-binding domain (NBD) were altered. Notable structural changes were observed for important residues within the Walker B motif that immediately follow pSer295. A favorable electrostatic environment was created for two residues (Lys232 and His522) that interact with ADP. Several other basic residues could establish favourable charge-charge interactions with the dianionic phosphate of pSer295. Arg296 and Glu343 underwent a functional change from negative/stabilizing to positive/destabilizing interaction upon phosphorylation of Ser295. Taken together, the results suggest that local structural transformations within the NBD could have consequences on the catalytic efficiency of the enzyme and suppress nucleotide turnover.