Tubulin isotypes polymerise into sectioned microtubules that locally regulate protein binding
Tubulin isotypes polymerise into sectioned microtubules that locally regulate protein binding
Prakash, M.; Chew, Y.-M.; Ondruskova, D.; Miksatko, J.; Dodokova, A.; Krishnan, A.; Janke, C.; Cross, R. A.; Lansky, Z.; Braun, M.
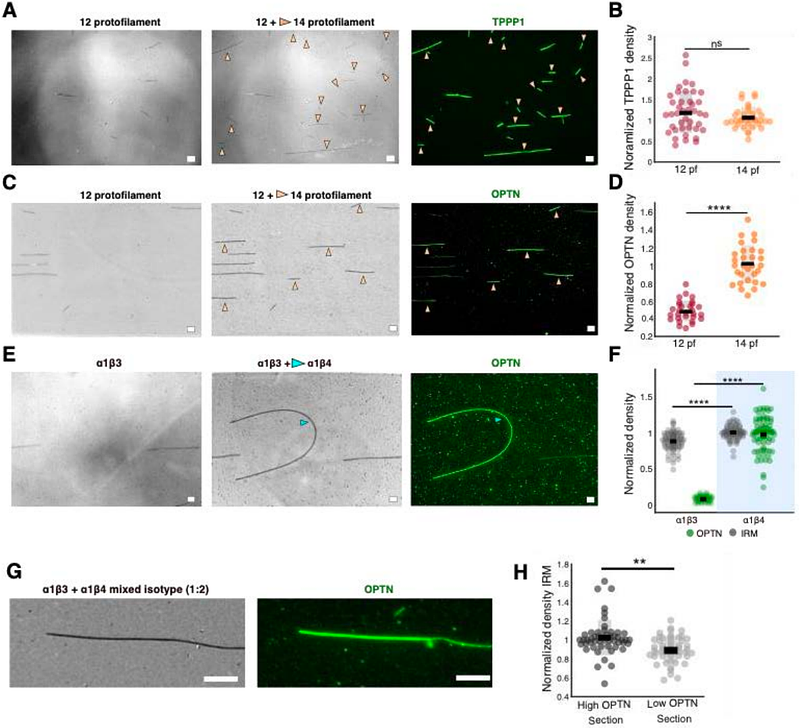
AbstractMicrotubules assemble from tubulin heterodimers composed of conserved - and {beta}- tubulins. In many species, including humans, tubulins are expressed from multiple genes. While the resulting tubulin isotypes show only subtle sequence differences, microtubules made of distinct isotypes can differ in their dynamic behaviour, as well as in their structure, for example in the number of their protofilaments. In cells, tubulin isotypes co-polymerize into mixed-isotype microtubules. How the mixing of tubulin isotypes affects microtubule functionality is unknown. Here we show that co-polymerization of recombinant tubulin dimers containing two different human {beta}-tubulin isotypes, 1{beta}3 and 1{beta}4, generates sectioned microtubules in which tubulin content and protofilament number differ from one section to the next. We demonstrate that two microtubule-associated proteins (MAPs), TPPP1 and optineurin (OPTN), bind differentially to these sections. Microtubules grown from natively mixed-isotype sources, either HeLa cells or mammalian brain tissue, also consist of sections that are differentially recognized by TPPP1 and OPTN, suggestive of isotype sorting. Our results demonstrate that co-polymerization of multiple tubulin isotypes can drive microtubules to assemble into distinct sections that locally regulate the interactions of MAPs with the microtubule lattice.