Single-stranded DNA binding proteins are essential components of the architectural LDB1 protein complex
Single-stranded DNA binding proteins are essential components of the architectural LDB1 protein complex
Wang, X.; Aboreden, N. G.; Cai, Y.; Lam, J. C.; Henderson, K. A.; Xiang, J.; Giardine, B. M.; Hardison, R. C.; Keller, C. A.; Nagarajan, L.; Brandt, S. J.; Blobel, G. A.
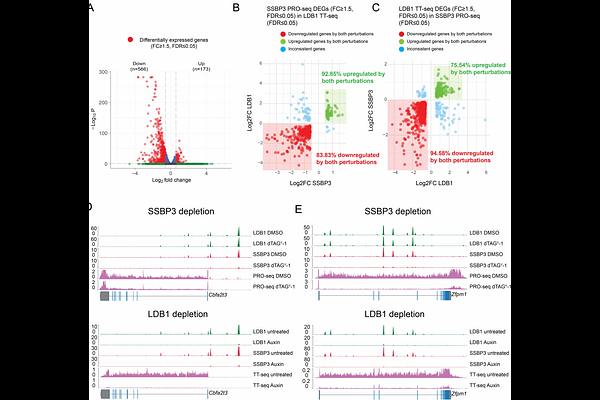
AbstractTranscriptional enhancers are brought into proximity with promoters via chromatin looping. The architectural transcription cofactor LDB1 facilitates spatial connectivity among enhancers and promoters but whether this occurs through simple dimerization or requires partner molecules is unknown. Here we investigated single-stranded DNA binding proteins (SSBPs), known LDB1 interactors, in regulating LDB1-mediated chromatin looping and transcription. SSBP2, SSBP3, and SSBP4 colocalize with LDB1 genome wide. Among these, only SSBP3 is essential for erythroid cell viability, LDB1 function, and transcription. LDB1, but not single-stranded DNA, is the predominant genome-wide tether of SSBP3 to chromatin. Notably, SSBP3 depletion for under one hour in SSBP2/4 knockout cells globally weakened LDB1-dependent chromatin loops and lowered nascent transcription without impacting LDB1\'s chromatin binding. Chromatin tethering experiments revealed SSBP3 and LDB1 mutually depend on each other to form looped contacts. SSBP3 stabilizes LDB1 homodimers in solution providing a possible mechanism of action. In sum, SSBPs emerge as key functional components of the architectural LDB1 complex, shedding new light on the regulation of enhancer-promoter interactions and gene expression.