Positive cooperativity between RAS-binding and cysteine-rich domains regulates RAF membrane binding kinetics via lateral rebinding
Positive cooperativity between RAS-binding and cysteine-rich domains regulates RAF membrane binding kinetics via lateral rebinding
Jimenez Salinas, A.; Tevdorashvili, K.; Grim, J.; Morales, A.; Chakhrakia, A.; Lee, Y. K.
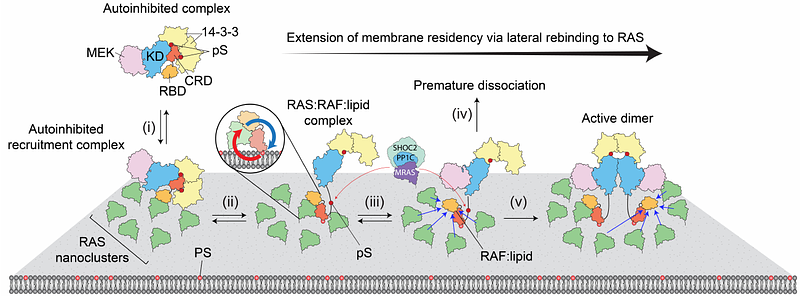
AbstractRAF activation requires interactions with both RAS nanoclusters and membrane lipids, yet the molecular basis of this process remains unclear. Using a bottom-up reconstitution approach, we show how coordinated protein-protein and protein-lipid interactions regulate membrane binding dynamics of RAF to drive its multistep activation. Within membrane environments, the RAS-binding domain (RBD) and cysteine-rich domain (CRD) exhibit cooperativity, with CRD-mediated phosphatidylserine binding stabilizing the RBD:RAS complex. Importantly, RAF remains membrane-bound through lateral rebinding to RAS, where a weak CRD-lipid interaction plays an essential role. This lateral rebinding extends membrane dwell time of RAF under high RAS density conditions, which are found in RAS nanoclusters. This prolonged membrane residence likely facilitates kinetic proofreading of RAF multistep activation within RAS nanoclusters, ensuring signaling specificity. Given the high abundance of weak multivalent membrane interactions, lateral rebinding may be a common mechanism for regulating the activity of signaling proteins through sustained membrane retention.