TRIM28 regulates pre-mRNA splicing via phosphorylation and SUMOylation networks
TRIM28 regulates pre-mRNA splicing via phosphorylation and SUMOylation networks
Matkovic, V.; Prieto-Garcia, C.; Mosler, T.; Wagner, K.; Hotz, P.; Haidle, F.; Schimmel, J.; Blumel, N.; Galej, W. P.; Vertegaal, A. C. O.; Muller-McNicoll, M.; Zarnack, K.; Muller, S.; Dikic, I.
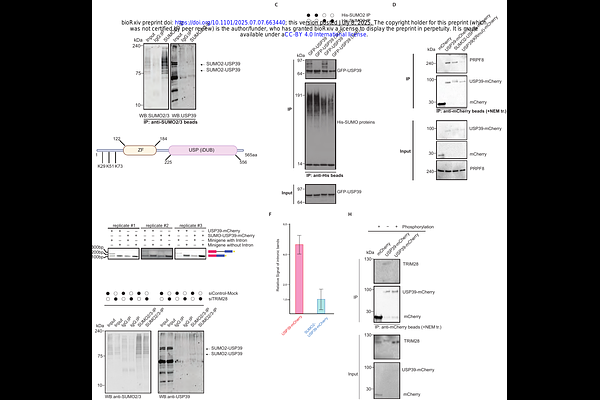
AbstractPre-mRNA splicing is a highly regulated process orchestrated by splicing factors, cis-acting elements, and interconnected cellular processes such as transcription and chromatin remodeling. Here, we identify a novel regulatory axis involving phosphorylation and SUMOylation that governs the function of Tripartite motif-containing 28 protein (TRIM28) and its role in pre-mRNA splicing. We demonstrate that TRIM28 interacts with the spliceosomal protein USP39 in a phosphorylation-dependent manner, with non-phosphorylated TRIM28 promoting USP39 SUMOylation at defined lysine residues. This post-translational modification enhances USP39\'s role within the U4/U6.U5 tri-snRNP complex. Functionally, TRIM28 knockdown induces widespread alterations in alternative splicing patterns, underscoring its importance in splicing regulation. Together, our findings uncover a mechanistic link between TRIM28-mediated post-translational modifications and the modulation of spliceosomal activity, offering new insights into how splicing decisions are integrated with cellular signaling pathways.