Single-molecule microscopy reveals that TFIIE subunits dynamically interact with preinitiation complexes in a manner controlled by TFIIH
Single-molecule microscopy reveals that TFIIE subunits dynamically interact with preinitiation complexes in a manner controlled by TFIIH
Archuleta, S. R.; Miller, R. C.; Mirita, J. A.; Goodrich, J. A.; Kugel, J. F.
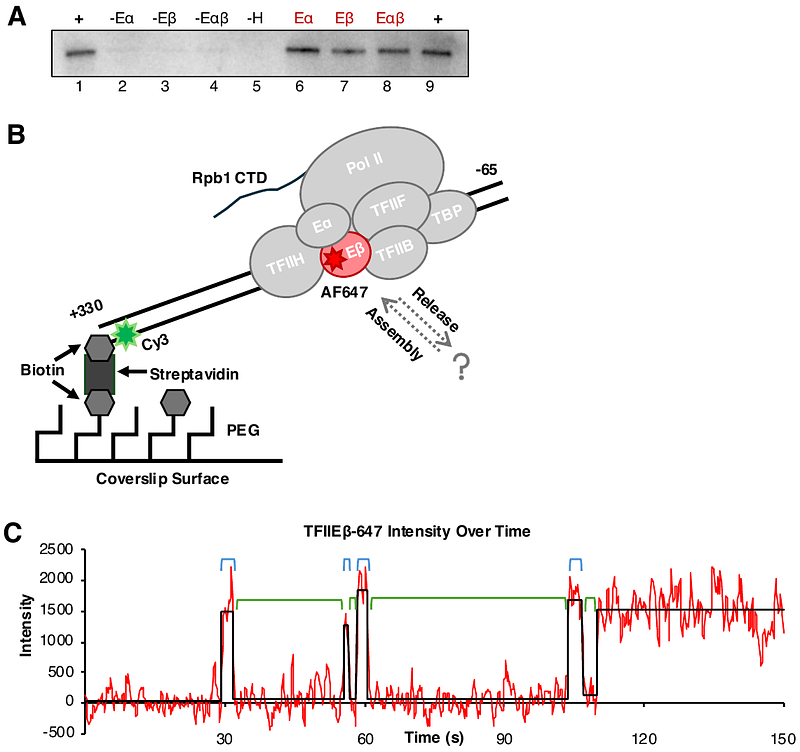
AbstractTranscription by RNA polymerase II (Pol II) requires general transcription factors that bind with Pol II at the promoters of protein-coding genes to form preinitiation complexes (PICs). Among these is TFIIE, which recruits TFIIH to the PIC and stimulates the kinase and translocase activities of TFIIH, thereby regulating the fate of formed PICs. In this study, we used a purified reconstituted human Pol II transcription system and single molecule total internal reflection fluorescence (smTIRF) microscopy to monitor TFIIE binding dynamics in PICs under different conditions in real time. We observed highly dynamic interactions of the two subunits of TFIIE (TFIIE and TFIIE{beta}) with PICs. Measurement of rate constants for on/off binding of each subunit suggest they behave asynchronously. TFIIH exclusion increased the rates of association and dissociation for both subunits, with the strongest effect on TFIIE. Despite stabilization of TFIIE by TFIIH the TFIIE subunits remain dynamic in PICs. Additionally, two disease-related TFIIE{beta} point mutations destabilized TFIIE{beta} and altered its kinetic behaviors within PICs. Our results contribute to an emerging model that PICs are not static assemblies and highlight important connections between the structural arrangement and kinetic behaviors of GTFs in PICs.