Integrative Transcriptomic and Machine Learning Approaches to decipher Mitochondrial Gene Regulation in severe Plasmodium vivax Malaria
Integrative Transcriptomic and Machine Learning Approaches to decipher Mitochondrial Gene Regulation in severe Plasmodium vivax Malaria
Roy, P.; Aggarwal, Y.; Kochar, S. K.; Kochar, D. K.; Das, A.
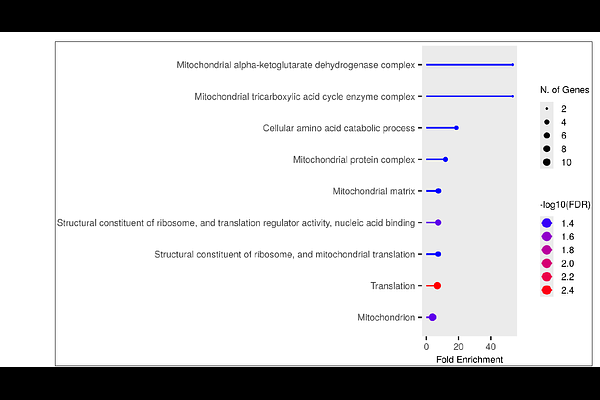
AbstractMitochondria in Plasmodium vivax are functionally vital despite possessing a highly reduced genome and differing substantially from the human organelle. Beyond their classical role in energy production, they dynamically coordinate processes like pyrimidine biosynthesis and heme metabolism, adapting their functions across the intra-erythrocytic development cycle (IDC). Their unique architecture and stage-specific roles enable the parasite to fine-tune mitochondrial gene expression, which operationally includes both sense and Natural Antisense Transcripts (NATs) - a class of long non-coding RNAs. The study involves the analysis of transcriptomic data to identify significant differentially expressed genes, in both sense and NATs categories, associated with severe malaria manifestations. This emphasizes the critical role of mitochondrial gene regulation in disease severity. These genes were statistically ranked and then used as input features for machine learning analysis for verification. Machine learning acted as a hypothesis-testing framework, enabling refinement of gene lists and strengthening biological interpretations. Further, a comprehensive gene enrichment analysis was performed for both sense and NATs to investigate the mitochondrial or other cellular pathways impacted during severe malaria. The findings revealed that NATs have a striking association with mitochondrial pathways and translation machinery, indicating that NATs are not merely by-products of transcription but also play a regulatory role in fine-tuning mitochondrial gene expression with severe manifestation. This work highlights mitochondrial NATs as critical regulators of parasite biology and positions the Plasmodium mitochondrion as a promising target for antimalarial drug development and therapeutic intervention.