Spatial control of karyopherin binding avidity within NPC mimics revealed by designer FG-Nucleoporins
Spatial control of karyopherin binding avidity within NPC mimics revealed by designer FG-Nucleoporins
de Vries, H. W.; Barth, A.; Fragasso, A.; Otto, T. A.; van der Graaf, A.; van der Sluis, E. O.; van der Giessen, E.; Veenhoff, L. M.; Dekker, C.; Onck, P. R.
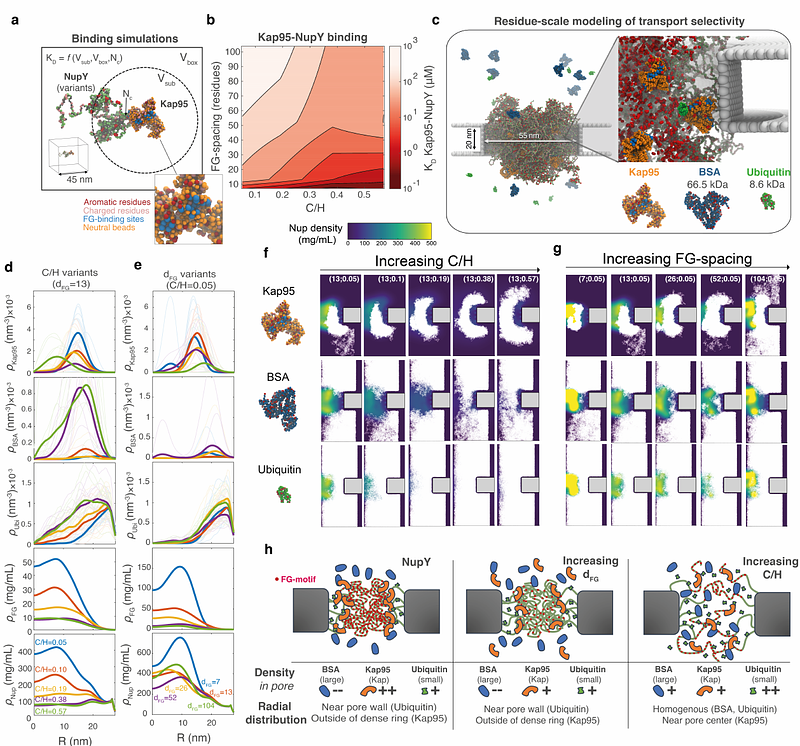
AbstractNucleocytoplasmic transport occurs via nuclear pore complexes (NPCs), ~40-60 nm wide pores lined with intrinsically disordered proteins that are rich in Phe-Gly motifs (FG-Nups) that form a selective barrier. Molecules larger than ~50 kDa are increasingly blocked for transport unless they are bound to a nuclear transport receptor (NTR). How the amino acid sequence of FG-Nups contribute to this is not fully understood. Here, we present de novo designed artificial FG-Nups with a systematically varied FG-repeat spacing and charge-to-hydrophobicity ratio (C/H). Starting from a reference sequence termed 'NupY' (with the average properties of natural yeast GLFG-Nups), we designed, synthesized, and experimentally tested a library of NupY variants using QCM-D experiments and phase separation assays. We find that the spacing between FG-motifs governs Kap95 absorption into the FG-Nup phase, while increasing C/H results in higher avidity for Kap95 due to an increased accessibility of FG-motifs. Molecular dynamics simulations of transport through NupY-coated pores show a reduced barrier function for noncohesive high-C/H-ratio variants and the highest transport selectivity for designs close to native GLFG-Nups. We postulate that a balance between entropic repulsion and enthalpic gain from multivalent Kap-FG-Nup interactions drives the spatial and temporal partitioning of Kaps in the NPC.