Tissue-specific restriction of TE-derived regulatory elements safeguards cell-type identity
Tissue-specific restriction of TE-derived regulatory elements safeguards cell-type identity
Milovanovic, D.; Duc, J.; Matsushima, W.; Hamelin, R.; Planet, E.; Offner, S.; Rosspopoff, O.; Trono, D.
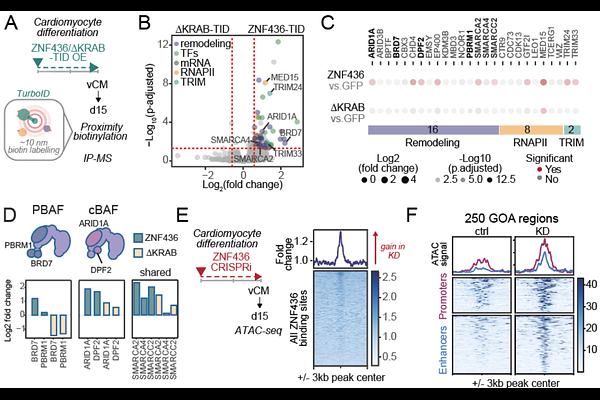
AbstractTransposable elements (TEs) have extensively reshaped the cis-regulatory landscape of mammalian genomes, yet the mechanisms that govern their context-specific activity remain incompletely understood. KRAB zinc finger proteins (KZFPs), a large family of transcription factors specialized in TE recognition, are known repressors of TE-derived regulatory activity through TRIM28-mediated H3K9me3 deposition. Here, we expand this paradigm by uncovering noncanonical relationships between TEs and KZFPs. By generating a comprehensive epigenomic map of KZFP-bound TEs, we find that the regulatory activity of ancient mammalian L2/MIR elements is broadly delineated by KZFP binding patterns, despite low H3K9me3 enrichment. We further dissect this relationship by investigating the function of ZNF436, a non-canonical KZFP, highly expressed during in vivo human fetal heart development. Using loss-of-function approaches, we show that ZNF436 preserves cardiomyocyte function by promoting cardiac gene expression while restricting the activation of alternative lineage programs. Mechanistically, ZNF436 recruits specialized SWI/SNF chromatin remodeling complexes to limit the accessibility of L2/MIR-derived enhancers, many of which are active in non-cardiac tissues. These findings reveal a noncanonical, TRIM28-independent role for KZFPs in shaping cell-type-specific regulatory landscapes and emphasize the importance of repressing alternative regulatory programs alongside activating lineage-specific ones to safeguard cell identity.