FDA drug repurposing uncovers modulators of dopamine D2 receptor localization via disruption of the NCS-1 interaction
FDA drug repurposing uncovers modulators of dopamine D2 receptor localization via disruption of the NCS-1 interaction
Munoz-Reyes, D.; Aguado, L.; Arroyo-Urea, S.; Requena, C.; Perez-Suarez, S.; Sanchez-Yepes, S.; Argerich, J.; Miro-Rodriguez, C.; Ulzurrun, E.; Rodriguez, E.; Garcia-Nafria, J.; Campillo, N. E.; Mansilla, A.; Sanchez-Barrena, M. J.
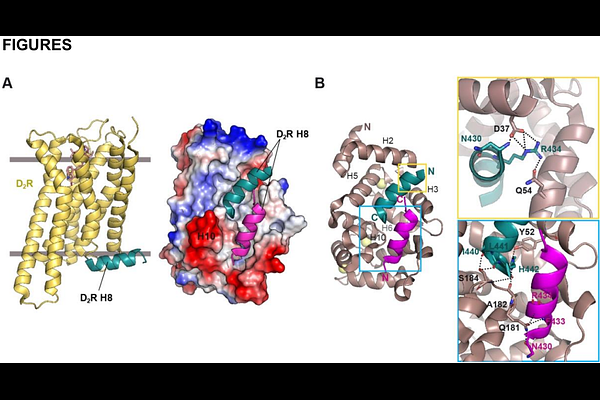
AbstractDopamine D2 receptor (D2R) regulates key aspects of motor control, cognition, and reward. Its function depends not only on ligand binding and signaling efficacy, but also on the dynamic control of receptor localization at the cell surface. Neuronal Calcium Sensor 1 (NCS-1) is a calcium binding protein which directly interacts with D2R in a Ca2+-dependent manner. Here, we investigated the regulatory role of NCS-1 in D2R localization and function. We found that NCS-1 promotes the trafficking of D2R to the plasma membrane through a mechanism dependent on active exocytosis. Functional signaling assays confirmed that NCS-1 does not alter the canonical receptor pharmacology. Using a library of FDA-approved drugs, a structure-based drug-repurposing strategy was designed to find protein-protein interaction modulators that allowed the exploration of the NCS-1/D2R interface as a new pharmacological target. Azilsartan medoxomil, atorvastatin, and vilazodone disrupted its interaction with D2R, reducing receptor surface expression in cells. Crystallography and molecular dynamics simulations revealed their mechanism of action. These compounds target the NCS-1 hydrophobic crevice and overlap the D2R binding site, perturbing the dynamics of the regulatory helix H10 in NCS-1. These findings uncover a previously unexploited intracellular mechanism for modulating D2R function and highlight the potential of targeting protein-protein interactions for therapeutic purposes. Our results provide a framework for fine-tuning dopaminergic tone through receptor localization mechanisms, offering an alternative strategy to conventional approaches based on receptor blockade or direct agonism