AT vs. GC binding of protamine-template: A microscopic understanding through molecular dynamics and binding free energies
AT vs. GC binding of protamine-template: A microscopic understanding through molecular dynamics and binding free energies
Mandal, S.; Chhetri, K. B.; Jang, Y. H.; Lansac, Y.; Maiti, P. K.
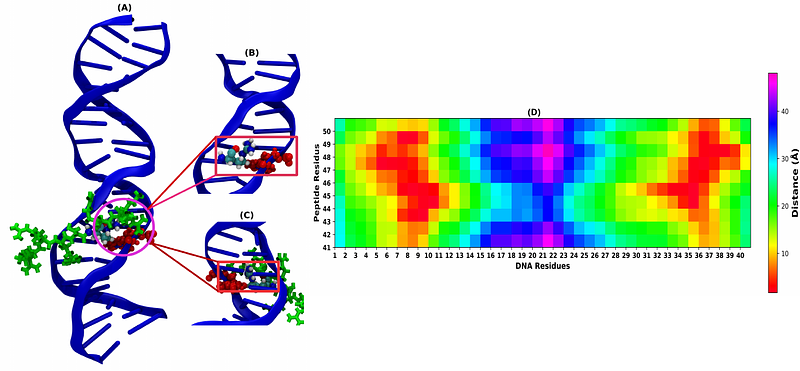
AbstractProtamine, an arginine-rich protein, compacts DNA more tightly than histones in somatic cells, yet its sequence-specific binding remains unclear. Using all-atom MD simulations with an arginine-rich short cationic peptide that mimics the protamine characteristics, we discovered distinct sequence preferences: the peptide binds preferentially to GC-rich sequences in the major groove and AT-rich sequences in the minor groove. Our structural analysis reveals that GC-rich binding induces significant DNA bending, narrowing the major groove, and enhancing peptide interactions. In contrast, AT-rich minor grooves are more extended and electronegative, allowing better stereochemical fitting with planar and aromatic guanidinium side groups of arginine. However, thymine\'s methyl group hinders major groove binding, favoring guanine. Thermodynamic free energy calculations, including MMGBSA, Jarzynski\'s Equality, and Umbrella Sampling, confirm stronger peptide affinity for AT-rich motifs in the minor groove and GC-rich motifs in the major groove. Overall, this study will enhance our understanding of DNA condensation and compaction in sperm cells based on protamine\'s sequence-specific interactions.