Huib32: A Potent and Selective USP32 Inhibitor Modulating Endosomal Processes and Advancing Cell-Permeable USP32 Probes
Huib32: A Potent and Selective USP32 Inhibitor Modulating Endosomal Processes and Advancing Cell-Permeable USP32 Probes
Scherpe, S.; Pol, V.; Kooij, R.; Pinto-Fernandez, A.; Tjokrodirijo, R. T. N.; O'Brien, D. P.; Vendrell, I.; Hameed, D. S.; van Veelen, P. A.; Kessler, B. M.; Geurink, P. P.; Sapmaz, A.
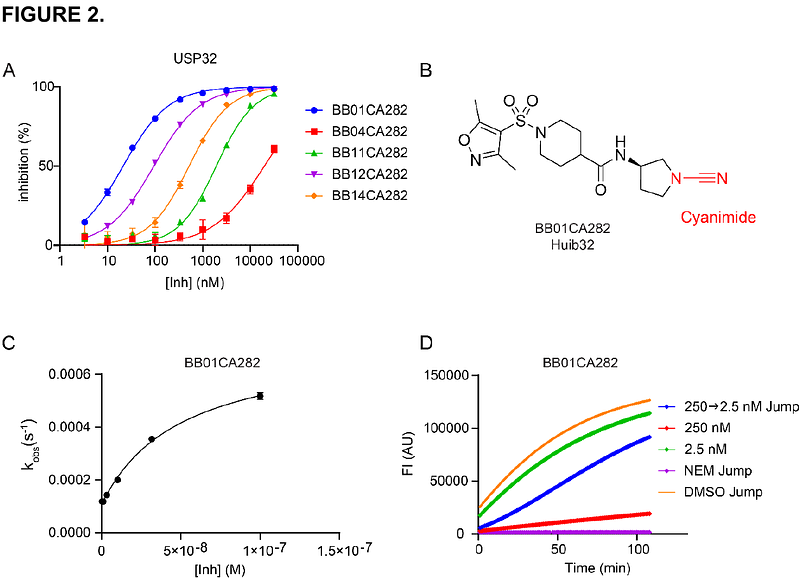
AbstractDeubiquitinating enzymes (DUBs) are pivotal regulators of ubiquitination, a vital post-translational modification essential for cellular processes. Dysregulated DUB activity disrupts cellular homeostasis, driving diseases like cancer and neurodegeneration. Ubiquitin-specific protease 32 (USP32) has emerged as a promising therapeutic target due to its role in endosomal and autophagosomal dynamics and its association with breast, ovarian, and lung cancers. Here, we describe Huib32 (Human deUbiquitinase Inhibitor 32) as a USP32 inhibitor. Cyanimide-containing Huib32 potently and selectively inhibits USP32 by covalently binding to the active site Cys743 in vitro and in cells, enhancing substrate ubiquitination, altering endosomal morphology, and mimicking USP32 depletion. Additionally, we present two activity-based probes (ABPs), Huib32*1 and Huib32*2, which enable precise detection of USP32 activity and confirm probe selectivity via mass spectrometry. Together, Huib32 and its probes represent a unique approach for targeting USP32, offering new research tools and potential therapeutic avenues for cancer and disorders involving endocytic trafficking.