Engineering lung-sensing T cells using synthetic receptors targeting RAGE
Engineering lung-sensing T cells using synthetic receptors targeting RAGE
Jacob, A.; Whitty, C.; Lupica, G.; Badwal, E.; Ren, X.; Qiu, W.; Yu, W.; Tonai, Y.; Camara Serrano, J. A.; Simic, M.; Lim, W. A.; Allen, G.; Sheppard, D.
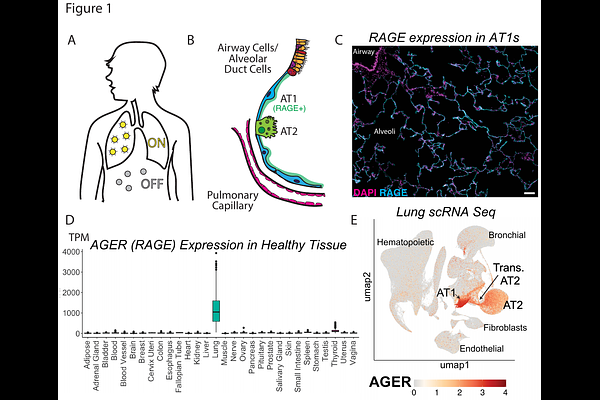
AbstractOur goal was to leverage synthetic biology approaches to engineer lung-sensing T cells that trigger synthetic transcriptional programs only when in the lung. First, we identified a lung-specific cell surface protein, receptor for advanced glycation endproducts (RAGE), expressed at high levels exclusively in the lung. Then, we engineered chimeric antigen receptors (CAR) and synthetic notch receptors (SynNotch) to bind this protein. We showed that anti-RAGE CAR-T cells traffic to and proliferate in the lung exclusively. Anti-RAGE SynNotch receptors activate transcription of a fluorescent reporter only when co-cultured with RAGE+ cell lines or primary lung cells. Finally, we tested an anti-RAGE SynNotch to anti-CD19 CAR circuit in vivo in mice implanted with lung and flank tumors and found that this approach cleared CD19-expressing lung tumors without affecting CD19-expressing flank tumors. Thus, we demonstrate that RAGE is a lung-specific target and that T cells expressing anti-RAGE receptors can sense and activate lung-specific therapeutic transcriptional programs. This approach could be extended to allow cell-based therapies that target and deliver genetically encoded payloads to treat lung diseases while avoiding systemic toxicity.