Energetic profiling reveals thermodynamic principles underlying amyloid fibril maturation
Energetic profiling reveals thermodynamic principles underlying amyloid fibril maturation
Duran-Romana, R.; Schymkowitz, J.; Rousseau, F.; Louros, N. N.
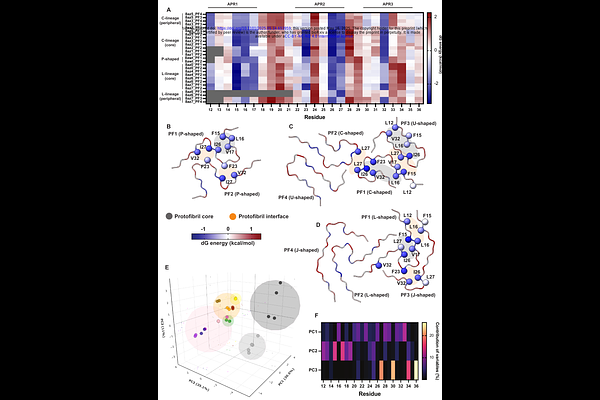
AbstractAmyloid fibrils adopt diverse structural polymorphs that underlie disease-specific phenotypes, but the thermodynamic principles guiding their formation and maturation remain poorly understood. Here, we apply energetic profiling to structural time series from cryo-EM datasets of IAPP, tau, and -synuclein to decode the principles governing fibril maturation, stability and polymorphic divergence. By mapping residue-level free energy contributions across experimentally resolved assembly pathways, we reconstruct the complex maturation trajectories of distinct amyloid systems. We find that amyloid assembly is driven by aggregation-prone regions that act as sequence-encoded stabilizing motifs. As assembly progresses, these motifs are reorganized and expanded, while additional regions introduce structural frustration that enables conformational flexibility. In several cases, environmental cofactors such as metal ions or polyanions are observed in association with regions of structural remodeling, where they may act to compensate for otherwise energetically strained conformations. Notably, structurally distinct polymorphs can exhibit similar energetic profiles, while polymorphs with similar folds may differ thermodynamically depending on their assembly environment. This framework connects polymorph structure to both encoded sequence features and extrinsic modifiers, offering mechanistic insight into how amyloid strains mature and diversify over time.