Cryo-EM insight into hydrogen positions and water networks in photosystem II
Cryo-EM insight into hydrogen positions and water networks in photosystem II
Hussein, R.; Graca, A. T.; Forsman, J.; Aydin, O. A.; Hall, M.; Gaetcke, J.; Chernev, P.; Wendler, P.; Dobbek, H.; Messinger, J.; Zouni, A.; Schroder, W. P.
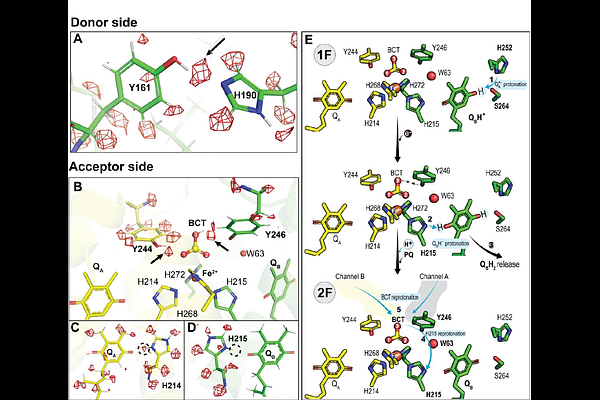
AbstractPhotosystem II starts the photosynthetic electron transport chain that converts solar energy into chemical energy and thereby sustains life on Earth. It catalyzes two chemical reactions, plastoquinone reduction and water oxidation to molecular oxygen, which both are performed at sequestered sites. While it is known that proton-coupled electron transfer is crucial for these processes, the molecular details have remained speculative due to incomplete structural data. Thus, we collected high-resolution cryo-EM data of photosystem II from Thermosynechococcus vestitus. The advanced structure (1.71 A) reveals several previously undetected occupied water binding sites and more than half of the hydrogen and proton positions of the protein. This unprecedented insight into the structure of photosystem II significantly enhances our understanding of its intricate protein-water-cofactor interactions enabling solar-driven catalysis.