Temporal and spatial profiling of ALKBH5 activity through NAIL-MS and compartmentalized RNA Isolation
Temporal and spatial profiling of ALKBH5 activity through NAIL-MS and compartmentalized RNA Isolation
Wesseling, H.; Kaiser, S.
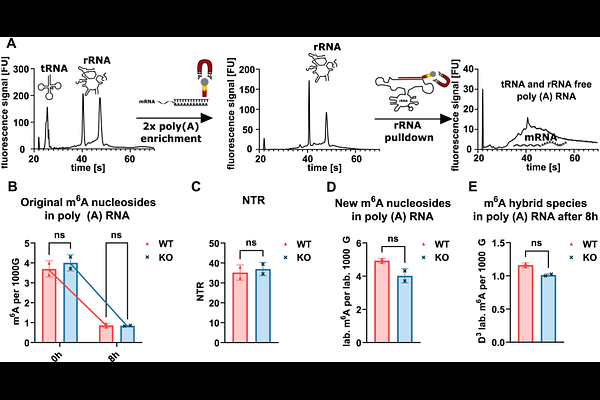
AbstractRNA modifications, especially m6A in human mRNA, are believed to be dynamically regulated through RNA writers and erasers. The key eraser of m6A is ALKBH5 with its function well proven in vitro, while in vivo evidence is lacking. Here, we set out to exploit nucleic acid isotope labelling coupled mass spectrometry (NAIL-MS) in a pulse chase set-up to study the in vivo function of ALKBH5 on human RNAs. For this we purified poly(A) from whole cell total RNA and found that, steady-state m6A levels and turnover dynamics were nearly identical between WT and ALKBH5 KO, despite clear evidence of robust RNA turnover within an 8-hour labeling period. To assess whether ALKBH5 might act in a compartment-specific manner, we employed an advanced subcellular fractionation strategy, allowing for the isolation of chromatin-associated, nucleoplasmic, and cytoplasmic RNA. These analyses confirmed that m6A accumulates during transcript maturation, with levels peaking in nuclear fractions and decreasing following export to the cytoplasm, supporting the thesis m6A is a dynamic modification. Notably, however, spatial and temporal profiles of m6A distribution and decay were unaffected by ALKBH5 KO. Even in chromatin-associated and nucleolar mRNA, where co-transcriptional modification and potential demethylation would be most plausible, m6A dynamics remained indistinguishable between WT and KO cells in the NAIL-MS context. We thus conclude that ALKBH5 has no major role in mRNA m6A demethylation in HEK 293T cells grown under optimal conditions.