WHEP Domain of Glycyl-tRNA Synthetase Regulates Neuropilin 1 Binding and Vascular Permeability
WHEP Domain of Glycyl-tRNA Synthetase Regulates Neuropilin 1 Binding and Vascular Permeability
Tong, Y.; Gioelli, N.; Zhang, H.; Dumitru, C. D.; Kanaji, S.; Horii, M.; Cheong, K. N.; Zhou, J.; Bolon, B.; Bai, G.; Serini, G.; Kanaji, T.; Yang, X.-L.
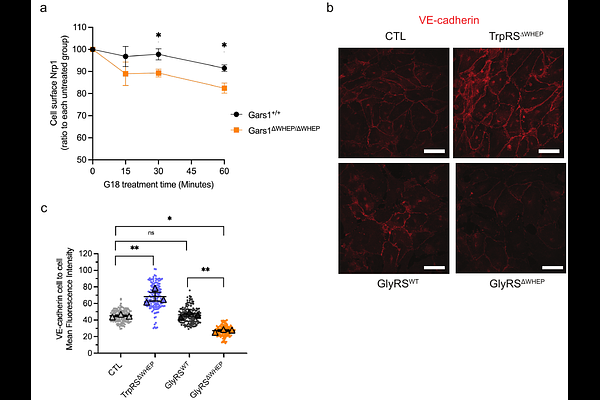
AbstractAminoacyl-tRNA synthetases (aaRSs) charge tRNAs with their cognate amino acids, ensuring accurate translation of the genetic code from mRNA to protein. During eukaryotic evolution, aaRSs acquired additional domains with unclear functions, including the WHEP domain, a two-helix bundle found in several eukaryotic aaRSs such as glycyl-tRNA synthetase (GlyRS, encoded by GARS1). We generated Gars1{Delta}WHEP mutant mice lacking exon 2, which disrupts most of the WHEP domain. Homozygous Gars1{Delta}WHEP/{Delta}WHEP mice exhibited late embryonic or neonatal lethality, with delayed lung development, characterized by reduced airway dilation (inflatability), and increased vascular leakage. Disruption of the WHEP domain did not impair tRNA aminoacylation but inhibited the free release of GlyRS from cells. Instead, GlyRS{Delta}WHEP was found in the membrane fractions and showed a stronger interaction with the extracellular region of neuropilin 1 (Nrp1) receptor compared to full-length GlyRS. This aberrant interaction enhanced Nrp1\'s endocytic activity and significantly reduced the localization of the Nrp1 interactor VE-cadherin at the adherens junctions of endothelial cells. A heterozygous knockout of Nrp1 in the Gars1{Delta}WHEP/{Delta}WHEP mice partially rescued body weight and vascular permeability defects. This study establishes a physiological role for the GlyRS WHEP domain in lung development and its regulation of GlyRS-Nrp1 interaction and vascular permeability.