Folding and knotting of biotic and pre-biotic amino acid sequences through reverse evolution
Folding and knotting of biotic and pre-biotic amino acid sequences through reverse evolution
Especial, J. N.; Faisca, P. F.
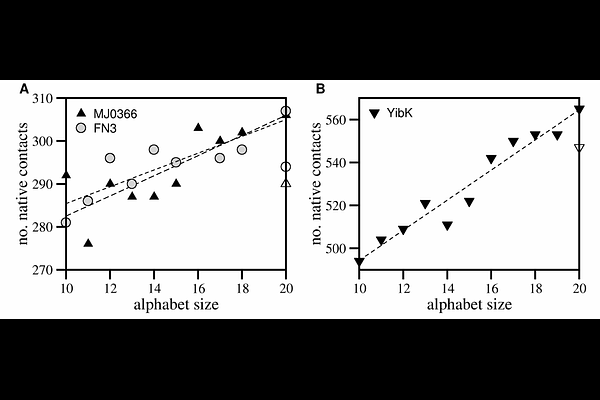
AbstractWe develped a simple reverse evolution method to explore the protein folding transition and knotting process in globular proteins throughout evolution using as a proxy for evolutionary time the lenght of the amino acid alphabet. Three small proteins were considered. An unknotted one featuring a beta-sandwhich fold (FN3), a protein embedding a shallow trefoil knot (MJ0366), and a deeply knotted trefoil protein (YibK). Results from Monte Carlo simulations of a native-centric Calpha model show that thermal stability increases througout evolution for all considered proteins suggesting it provided a selective pressure for the introduction of biosynthetic amino acids in the alphabet repertoire. Additionally, the thermodynamic cooperativity, the two-state nature of the folding transition, and the kinetic efficiency of folding (and knotting) displayed by the unknotted (and shallow knotted) protein have been roughly conserved throughout evolution, indicating that early alphabets, and, in particular the anscestral one composed of 10 amino acids, is competent to self-assemble relatively complex native structures. However, while the ancestral alphabet is clearly more efficient in collapsing the polypetpide chain to non-specific (i.e. non-native) knots, it significantly compromises the thermodynamic cooperativity and the folding ability of the deeply knotted protein YibK. The findings presented here collectively suggest that the alphabet of amino acids evolved to improve folding, but early alphabets might not have favoured the folding of native structures with deep knots. This could help to explain why deep knots in proteins are statistically uncommon.