Accurate and Fast Protein Acylation Identification by Eliminating Position Effects of Cyclic Immonium Ions with Stepped HCD
Accurate and Fast Protein Acylation Identification by Eliminating Position Effects of Cyclic Immonium Ions with Stepped HCD
Zhu, Z.-Y.; Mao, P.-Z.; Tarn, C.; Cao, Y.
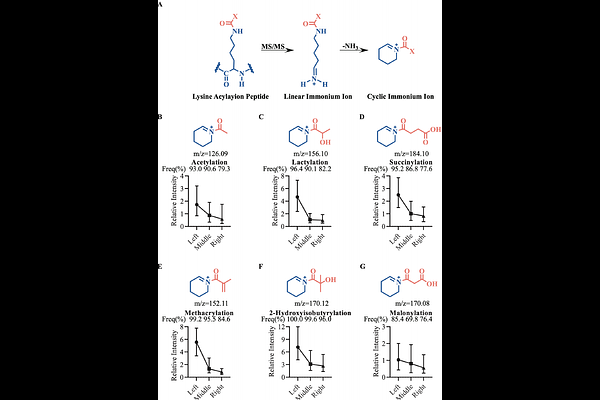
AbstractMass spectrometry (MS)-based proteomics is indispensable for studying post-translational modifications (PTMs). Cyclic immonium (CycIm) ions serve as invaluable diagnostic markers for lysine acylations, yet the principles governing their generation efficiency are poorly understood. Here, we systematically investigate this question and uncover a robust \"position effect\": the generation of immonium ions is strongly favored when the modified residue is located near the N-terminus of a tryptic peptide. Utilizing LysargiNase digestion and isotope-labeled synthetic peptides, we demonstrate that this effect is likely driven by the inherent instability of b-type fragment ions during collision-induced dissociation. Furthermore, we show that a stepped HCD strategy enables enhanced sequence coverage and robust CycIm ion detection (~99%), thereby improving the depth, reliability and speed of PTM identification. Collectively, this work provides fundamental understanding of immonium ion formation and establishes an optimized acquisition and analysis strategy that enhances the efficiency and confidence of PTM analysis.