Genetic Surveillance Reveals Differential Evolutionary Dynamic of Anopheles gambiae Under Contrasting Insecticidal Tools used in Malaria control
Genetic Surveillance Reveals Differential Evolutionary Dynamic of Anopheles gambiae Under Contrasting Insecticidal Tools used in Malaria control
Njoroge, H.; Namuli, L.; Nagi, S. C.; Hernandez-Koutoucheva, A.; McDermott, D. P.; Knight, E.; Gonahasa, S.; Lynd, A. R.; Oruni, A.; Maiteki-Sebuguzi, C.; Opigo, J.; Yeka, A.; Katureebe, A.; Kyohere, M.; Kamya, M.; Dorsey, G.; hemingway, J.; Staedke, S.; Clarkson, C. S.; Miles, A.; Lucas, E. R.; Donnelly, M. J.
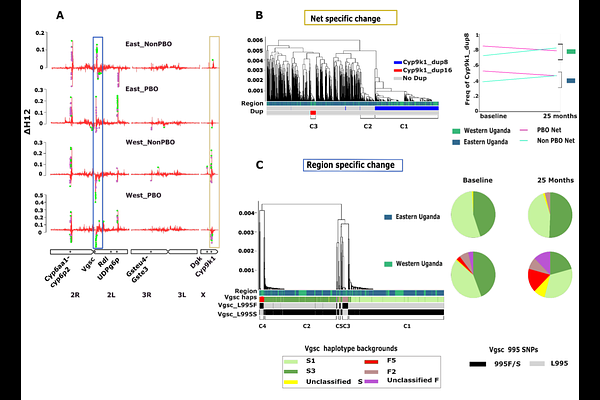
AbstractMalaria, a febrile disease caused by the Plasmodium parasites and transmitted by mosquitoes, is a leading cause of mortality in children under 5 in endemic countries. The widespread deployment of insecticide-treated bed nets (ITNs) has significantly reduced malaria transmission, but rising levels of insecticide resistance threatens to halt the progress. Monitoring insecticide resistance is vital for effective vector control, particularly when deploying new tools. Understanding mosquito population responses to these interventions is crucial for guiding control programmes in making informed decisions about the selection, timing, and geographic deployment of tools. This genomic study investigates the demographic and evolutionary consequences on the malaria vector Anopheles gambiae of deploying standard ITNs (containing only pyrethroids) and pyrethroid-PBO nets (containing pyrethroids plus the synergist piperonyl butoxide) during a clinical trial in Uganda. Despite substantial reductions in indoor mosquito densities in the clinical trial, estimates of nucleotide diversity ({pi}) and linkage disequilibrium revealed no significant decline in effective population size, reflecting continued large population size even after effective control. Marked allele frequency shifts at resistance-associated loci indicated strong selection pressures driven by the interventions, with distinct selective dynamics between the two net types, highlighting alternative pyrethroid detoxification pathways in the presence of PBO. A duplication in the Cyp9k1 gene significantly increased in frequency in populations exposed to pyrethroid-only nets but decreased in populations exposed to PBO-treated nets, suggesting that selection for over-expression of this gene is removed when this resistance mechanism is impacted by PBO. An alternative potential detoxification mechanism was selected within a region of the 2La chromosomal inversion on chromosome 2L, which encompasses the UDP-glucose 6-dehydrogenase gene. This variant consistently increased in frequency when exposed to PBO-treated nets. Additionally, pyrethroid-only nets selected for a novel locus on the X chromosome containing the diacylglycerol kinase gene, which is potentially linked to behavioural adaptations through its role in neurotransmission modulation. Our findings underscore the importance of genomic surveillance in vector control, revealing distinct evolutionary dynamics of insecticide resistance mechanisms in the presence of PBO. While ITNs remain effective, the persistence and evolution of resistance-associated alleles highlight the need for adaptive and dynamic resistance management strategies. By integrating high-resolution genomic data with epidemiological and entomological monitoring, this study offers actionable insights to sustain malaria control efforts amid the ongoing challenge of insecticide resistance.