The endoribonuclease Rae1 from Bacillus subtilis cleaves mRNA upstream of stalled ribosomes
The endoribonuclease Rae1 from Bacillus subtilis cleaves mRNA upstream of stalled ribosomes
Deves, V.; D'Halluin, A.; Gilet, L.; Condon, C.; Braun, F.
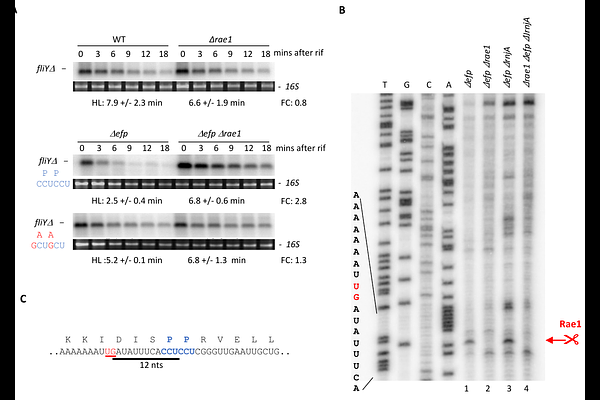
AbstractThe ribosome-associated endoribonuclease 1 (Rae1) cleaves mRNAs in a translation-dependent manner. Here, we identify a new Rae1 target, the fliY mRNA, which is cleaved by Rae1 in the absence of the elongation factor P (EF-P). The Rae1 site was mapped 12 nucleotides upstream of the second proline codon of an SPP stalling motif in fliY. Remarkably, Rae1 cleavages also occur 12 nucleotides upstream of the stop codon within two validated Rae1 mRNA targets, bmrX and spyA (S1025). Shifting the stop codon relative to the Rae1 cutting site abolished Rae1 sensitivity of bmrX and spyA mRNAs. We show that ribosome pausing occurs at the spyA stop codon, confirming its crucial role, and positioning the Rae1 cleavage at the tail end of the stalled ribosome, rather than in the A-site as previously proposed. These findings reveal a compelling novel mechanism by which Rae1 mediates mRNA cleavage in coordination with immobile ribosomes.