The NHSL1-A complex interacts with the Arp2/3 complex and controls cell migration efficiency and chemotaxis.
The NHSL1-A complex interacts with the Arp2/3 complex and controls cell migration efficiency and chemotaxis.
Jalal, S.; Pallett, T.; Wu, S.-y.; Asokan, S. B.; Bear, J. E.; Krause, M.
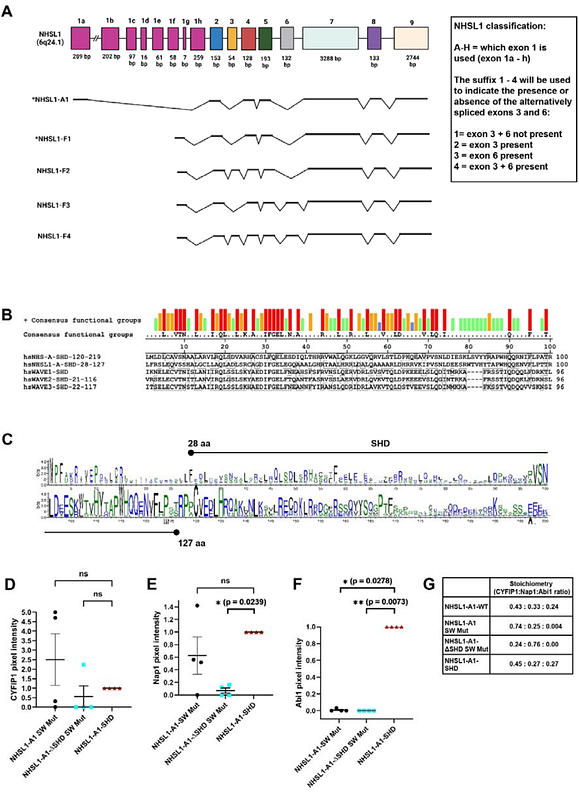
AbstractCell migration is crucial for development and deregulation causes diseases. The Scar/WAVE complex promotes mesenchymal cell migration through Arp2/3 mediated lamellipodia protrusion. We previously discovered that all isoforms of Nance-Horan Syndrome-like 1 (NHSL1) protein interact directly with the Scar/WAVE complex and the NHSL1-F1 isoform negatively regulates Scar/WAVE-Arp2/3 activity thereby inhibiting 2D random cell migration. Here, we investigate the NHSL1-A1 isoform, which contains a Scar homology domain (SHD). The SHD in Scar/WAVE mediates the formation of the Scar/WAVE complex. We found that the SHD of NHLS1-A is sufficient for the formation of an NHSL1-A complex composed of the same proteins as the Scar/WAVE complex, but NHSL1-A replaces Scar/WAVE. NHSL1-A SHD recruits the NHSL1-A complex to lamellipodia, where also the Scar/WAVE complex resides. Scar/WAVE contains a WCA domain, which is phosphorylated by CK2 and recruits and activates the Arp2/3 complex to nucleate branched actin networks supporting lamellipodial protrusion. We identified a WCA domain in NHSL1 which interacts with the Arp2/3 complex. The NHSL1 WCA domain is phosphorylated by GSK3, and this increases the interaction with the Arp2/3 complex. In contrast to NHSL1-F1, the NHSL1-A complex promotes cell migration speed but not cell persistence via the Scar/WAVE complex and potentially via its WCA domain. In addition, the NHSL1-A complex is required for chemotaxis. Mechanistically, the NHSL1-A complex may increase lamellipodial Arp2/3 activity and lamellipodial speed while reducing lamellipodial persistence. Our findings reveal an additional layer of Arp2/3 complex control essential for mesenchymal cell migration highly relevant for development and disease.