A viral SAVED protein with ring nuclease activity degrades the CRISPR second messenger cA4
A viral SAVED protein with ring nuclease activity degrades the CRISPR second messenger cA4
Orzechowski, M.; Hoikkala, V.; Chi, H.; McMahon, S.; Gloster, T.; White, M. F.
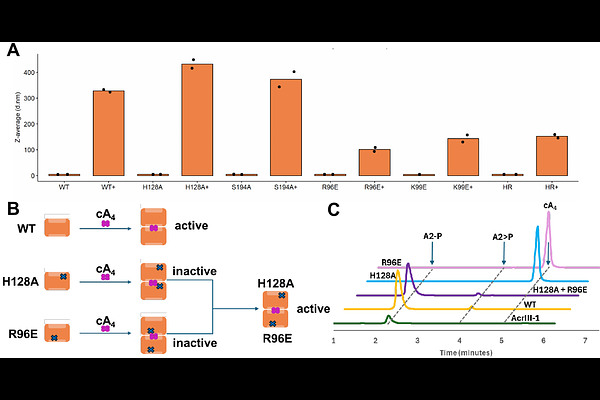
AbstractType III CRISPR systems typically generate cyclic oligoadenylate (cOA) second messengers such as cyclic tetra-adenylate (cA4) on detection of foreign RNA, activating ancillary effector proteins which elicit a diverse range of immune responses. The CalpLTS system elicits a transcriptional response to infection when CalpL binds cA4 in its SAVED (SMODS associated and fused to various effectors domain) sensor domain, resulting in filament formation and activation of the Lon protease domain, which cleaves the anti-Sigma factor CalpT, releasing the CalpS Sigma factor for transcriptional remodelling. Here, we show that thermophilic viruses have appropriated the SAVED domain of CalpL as an anti-CRISPR, AcrIII-2, which they use to degrade cA4. AcrIII-2 dimers sandwich cA4, degrading it in a shared active site to short linear products, using a mechanism highly reminiscent of CalpL. This results in inhibition of a range of cA4 activated effectors in vitro. This is the first example of a virally-encoded SAVED domain with ring nuclease activity, highlighting the complex interplay between viruses and cellular defences.