Model-independent reorganization of translation in TDP-43 Amyotrophic Lateral Sclerosis
Model-independent reorganization of translation in TDP-43 Amyotrophic Lateral Sclerosis
Lauria, F.; Maniscalco, F.; Perrucci, C.; Marchioretto, M.; Bruno, I.; Tome, G.; Cella, F.; Busarello, E.; Sevegnani, M.; Lunelli, L.; Donini, L.; Tebaldi, T.; D Antoni, M.; Pollini, D.; Peroni, D.; Pisciottani, A.; Croci, L.; Badaloni, A.; Arnese, R.; Provenzani, A.; Quattrone, A.; Consalez, G. G.; Clamer, M.; Siciliano, V.; Basso, M.; Viero, G.
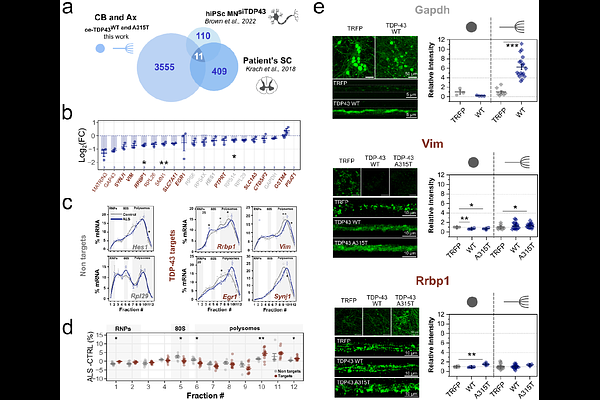
AbstractThe RNA-binding protein TDP-43 is a major contributor of Amyotrophic Lateral Sclerosis (ALS). Despite its recognized involvement in the disease, the molecular mechanisms linking TDP-43 dysregulation to ALS pathogenesis remain elusive. No clear consensus has been reached as to how it may directly or indirectly impact protein synthesis at genome-wide level. To elucidate TDP-43-associated dysfunctions in this process, we exploited in cellulo and in vivo models of TDP-43 proteinopathy integrating multiple ribosome profiling techniques, a tag-free miniaturized polysomal profiling coupled with RNA sequencing, and biochemical assays with subcellular (i.e., cell body and axon) and compartment-specific (i.e., RNA granules and polysomes) resolution. Across ALS models, we identified a systemic dysregulation in translation, characterized by: i) widespread axonal- and polysome-specific downregulation of TDP-43 target mRNAs, ii) reorganization of mRNA distribution between ribosome-free and polysomal compartments, and iii) altered ribosome dynamics and occupancy with downstream reorganisation of ribosome engagement with mRNAs. These findings suggest that ALS pathogenesis is characterised by a systemic reorganisation of ribosomes, providing a novel mechanistic framework for understanding disease progression.