Atypical features of the disordered N-terminal region of a bacterial outer membrane copper transporter.
Atypical features of the disordered N-terminal region of a bacterial outer membrane copper transporter.
Khochtali, A.; Ote, M.; Balon, H.; Dieu, M.; Renard, P.; Michaux, C.; Matroule, J.-Y.
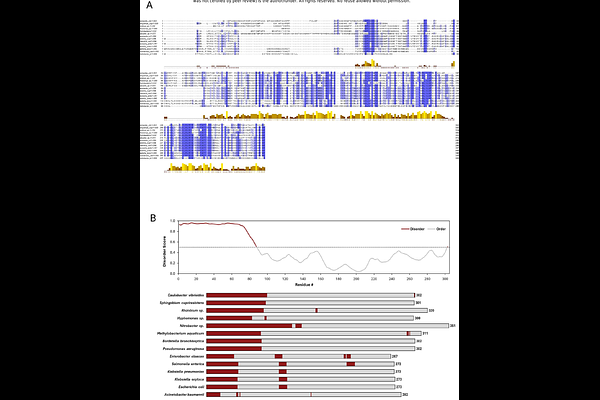
AbstractMetals like copper (Cu), zinc, and nickel exhibit dual nature, necessitating a tight regulation of their cellular homeostasis to meet physiological demands while preventing toxicity. In bacteria, metal homeostasis involves inner membrane (IM) P-type ATPases and ABC transporters, envelope-spanning tripartite efflux pumps, and outer membrane (OM) pore-forming proteins.\n\nFour decades ago, the OM {beta}-barrel protein PcoB was shown to provide an additional layer of Cu resistance in an Escherichia coli strain isolated from the gut of swine fed with Cu supplements. Interestingly, most PcoB homologs contain a poorly conserved disordered N-terminal domain (NTD) rich in histidine (His) and methionine (Met) residues, which are commonly associated with Cu coordination in cuproproteins. This suggests a potential role for the NTD in PcoB-mediated copper efflux.\n\nWe previously demonstrated that the free-living bacterium Caulobacter vibroides primarily relies on PcoB for Cu homeostasis. Here, we show that the NTD of C. vibroides PcoB is critical for PcoB function and stability, tolerating the swapping with the poorly conserved E. coli PcoB NTD and significant truncations. Unexpectedly, the predicted signal peptide (SP) was dispensable, challenging traditional concepts of protein translocation mechanisms. Moreover, the PcoB NTD plays a surprising role in stabilizing the periplasmic multicopper oxidase PcoA, encoded within the same operon as PcoB, highlighting a new role for an intrinsically disordered region (IDR).