Anionic Phospholipids Modulate Hepatitis C Virus Core Protein Assembly on Lipid Membranes
Anionic Phospholipids Modulate Hepatitis C Virus Core Protein Assembly on Lipid Membranes
Mandal, T.; Albrecht, B.; Chiantia, S.
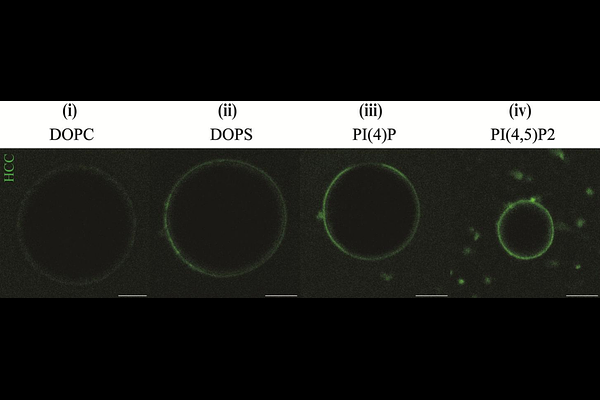
AbstractHepatitis C virus (HCV) genome codes for various proteins essential to its replication cycle. Among these, the core protein (HCC) forms the capsid via organised multimerization, thereby guiding viral assembly. This process depends on the dynamic localisation of HCC between the endoplasmic reticulum bilayer membrane and the lipid droplet monolayer. While past studies have examined the role of some HCC structural properties and other viral and host proteins in the assembly process, the contribution of lipid molecules was not thoroughly investigated yet. Since specific lipids are upregulated during HCV infection, it is reasonable that these molecules might play an important role for virus fitness and infection progression. In this study, we have addressed this topic and, specifically, how HCC-lipid interactions might impact HCC-HCC interactions. Using in vitro models and quantitative fluorescence microscopy techniques, we investigated HCC binding affinity to lipid membranes, multimerization and lateral diffusivity. Our results reveal the specificity of HCC interactions with anionic phospholipids (PLs) in terms of binding to monolayers and bilayers. Furthermore, our analysis shows that anionic PLs enhance HCC multimerization and, therefore, might drive lateral assembly for capsid formation. These results not only provide insights into the lipid-driven regulation of HCV assembly and identify anionic PLs as potential modulators of the viral replications cycle, but also highlight a previously unrecognised role of PL composition in HCC-membrane interaction.