Nucleotide and metalloid-driven conformational changes in the arsenite efflux ATPase ArsA
Nucleotide and metalloid-driven conformational changes in the arsenite efflux ATPase ArsA
Mahajan, S.; Pall, A. E.; Li, Y. E.; Stemmler, T. L.; Rees, D. C.; Clemons, W. M.
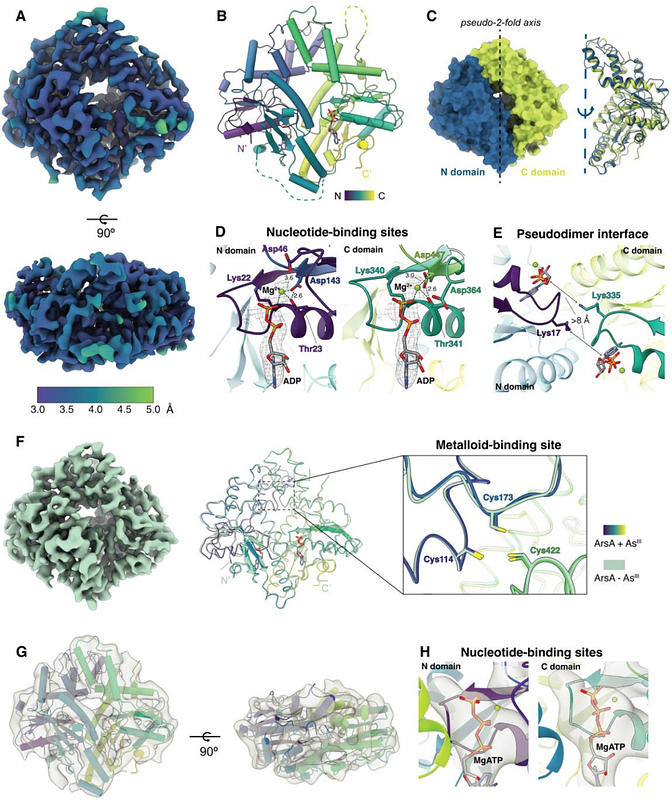
AbstractA common mechanism of arsenic detoxification in bacteria is arsenite (AsIII) efflux facilitated by the ArsAB pump that couples metalloid transport to ATP hydrolysis. The cytoplasmic ATPase component, ArsA, binds and hydrolyzes ATP and facilitates the transfer of AsIII to the integral membrane transporter, ArsB. The underlying molecular mechanism of AsIII efflux by ArsAB remains unclear. ArsA is a member of the Intradimeric Walker A (IWA) family of ATPases that undergo dramatic nucleotide-dependent conformational changes to facilitate their respective biological functions. Similar conformational transitions in ArsA have been postulated to drive AsIII binding and transport via ArsB but have not been demonstrated. Here, we report multiple structures of ArsA determined by single-particle cryogenic electron microscopy in an open MgADP-bound state, open MgATP-bound state, and a distinct closed MgATP-bound state liganded to AsIII. Using X-ray absorption spectroscopy, we confirmed that AsIII coordinates three conserved cysteines at the metalloid-binding site of the closed state in a three-coordinate fashion. Coupled with biochemical characterization, our cryo-EM structures reveal key conformational changes in the ArsA catalytic cycle consistent with other members of the IWA family and provide the structural basis for allosteric activation of nucleotide hydrolysis by AsIII. This work enhances our understanding of how the ArsA catalytic cycle regulates metalloid efflux by ArsB.