Coronary Artery Disease Risk Variant rs6903956 Links to Endothelial Dysfunction via PHACTR1 Regulation
Coronary Artery Disease Risk Variant rs6903956 Links to Endothelial Dysfunction via PHACTR1 Regulation
Tay, K. Y.; Wee, H. S.; Nguyen, N.; Autio, M. I.; Wazny, V. K.; Lee, K. L.; Tay, D.; Wang, Y.; Gao, X.; Heng, C. K.; Chan, M. Y.; Foo, R. S.; Lee, J.; Loh, M.; Cheung, C.
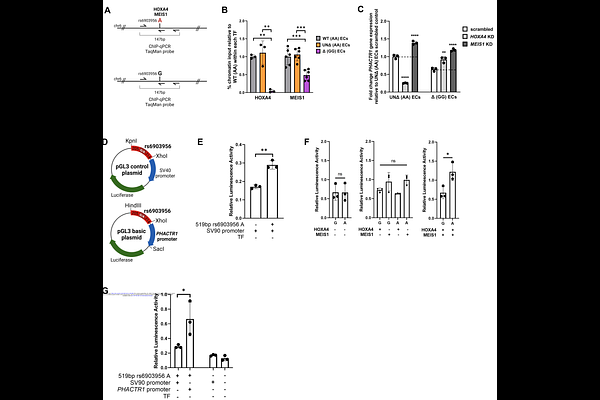
AbstractIschemic heart disease, particularly coronary artery disease (CAD), remain leading causes of mortality worldwide. The single nucleotide polymorphism rs6903956 on chromosome 6p24.1 has been identified as a susceptibility locus for CAD in East Asian populations through genome-wide association studies. However, its functional role has not been fully elucidated. This study investigates the mechanistic basis of rs6903956 and its contribution to CAD pathogenesis, focusing on endothelial cell dysfunction. We first conducted cohort studies, revealing an association between the rs6903956 risk allele A and blood pressure phenotypes, along with impaired endothelial responsiveness indicated by reduced flow-mediated dilation. Single-base editing of induced pluripotent stem cell-derived endothelial cells obtained from patients with CAD and expression quantitative trait loci analysis highlighted a cis-acting impact of the A allele on PHACTR1 and EDN1 expression, suggesting allele-specific regulatory effects. Using in silico modeling by AlphaFold 3 platform, the A allele exhibited enhanced binding affinity for HOXA4 and MEIS1 transcription factors, forming a stable ternary complex that promoted transcriptional activation of PHACTR1. Functional assays demonstrated the enhancer role of rs6903956 A allele in PHACTR1 promoter activity, supporting its locus-specific regulatory function in endothelial cells. Under pathological flow conditions, endothelial cells harboring the A allele display elevated ICAM-1 expression and increased monocyte adhesion compared to the G allele, indicating allele-specific endothelial inflammatory activation. These findings propose a model in which rs6903956 influences PHACTR1 expression via HOX-MEIS cooperative binding, thereby modulating endothelial function and contributing to CAD susceptibility. This study provides mechanistic insights into the role of rs6903956 in endothelial dysfunction and CAD, informing potential therapeutic targets arising from genetic determinants in cardiovascular pathogenesis.