Organism-wide cellular dynamics and epigenomic remodeling in mammalian aging
Organism-wide cellular dynamics and epigenomic remodeling in mammalian aging
Lu, Z.; Zhang, Z.; Xu, Z.; Abdulraouf, A.; Zhou, W.; Cao, J.
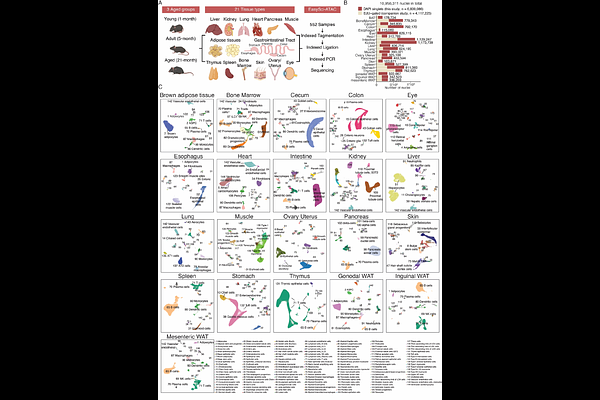
AbstractAging leads to functional decline across tissues, often accompanied by profound changes in cellular composition and cell-intrinsic molecular states. However, a comprehensive catalog of how the population of individual cell types change with age and the associated epigenomic dynamics is lacking. Here, we constructed a single-cell chromatin accessibility atlas consisting of ~7 million cells from 21 tissue types spanning three age groups in both sexes. This dataset revealed 536 main cell types and 1,828 finer-grained subtypes, defined by unique chromatin accessibility landscapes at ~1.3 million cis-regulatory elements. We observed widespread remodeling of immune lineages, with increases in plasma cells and macrophages, and depletion of T and B cell progenitors. Additionally, non-immune cell populations, including kidney podocytes, ovary granulosa cells, muscle tenocytes and lung aerocytes, showed marked reductions with age. Meanwhile, many subtypes changed synchronously across multiple organs, underscoring the potential influence of systemic inflammatory signals or hormonal cues. At the molecular level, aging was marked by thousands of differentially accessible regions, with the most concordant changes shared across cell types linked to genes related to inflammation or development. Putative upstream factors, such as intrinsic shifts in transcription factor usages and extrinsic cytokine signatures, were identified. Notably, around 40% of aging-associated main cell types and subtypes showed sex-dependent differences, with tens of thousands of chromatin accessibility peaks altered exclusively in one sex. Together, these findings present a comprehensive framework of how aging reshapes the chromatin landscape and cellular composition across diverse tissues, offering a comprehensive resource for understanding the molecular and cellular programs underlying aging and supporting the exploration of targeted therapeutic strategies to address age-related dysfunction.